Radiopharmaceuticals
| Home | | Pharmaceutical Drugs and Dosage | | Pharmaceutical Industrial Management |Chapter: Pharmaceutical Drugs and Dosage: Radiopharmaceuticals
Radioisotopes, also called radionuclides, are usually artificially produced unstable atoms of a naturally occurring element.
Radiopharmaceuticals
Introduction
Radioisotopes,
also called radionuclides, are usually artificially produced unstable atoms of
a naturally occurring element. These isotopes have the same number of electrons
and protons as the naturally occurring element, but different number of
neutrons. More than 1000 radioisotopes are known to occur. Of these, only about
50 are naturally occurring. Most radioiso-topes are produced by bombarding the
atoms of the stable, naturally occur-ring element with fast-moving neutrons
produced in a nuclear reactor or particle accelerator. These isotopes tend to
revert to the natural, stable ele-ments at a rate that is specific to each
isotope of each element. The rate of conversion of an isotope to its stable
elemental composition determines its time in existence and is measured by
half-life, the time it takes for half of the radioisotope population to
convert.
In
a hospital setting, radiopharmaceuticals are typically handled by the nuclear
pharmacy or radiopharmacy, involved in the preparation of radio-active
materials for diagnosis and/or treatment of specific diseases. In diag-nostic
applications, radiopharmaceuticals accumulate in specific tissues or cells and
emit radiation, which can be collected and processed into images, showing the
location of the accumulation in the body, for diagnostic pur-poses. In
therapeutic applications, the high-energy radiation released by
radiopharmaceuticals destroys undesired local cells and tissue.
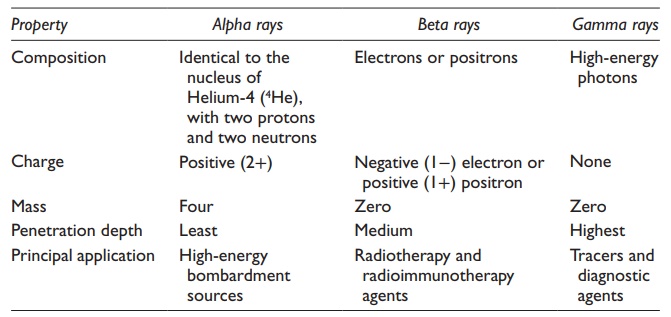
Types of radiation
The
unstable nuclei of radioisotopes dissipate energy, in the form of specific
types of radiation, as they spontaneously convert to the stable parent
iso-topes. These radiations are commonly known as alpha, beta, or gamma rays.
1. Alpha radiation is a result of excess energy dissipation
by unstable nuclei in the form of alpha particles. The alpha particles have two
positive charges and a total mass of four units. This is exemplified by
polonium 210Po84 decaying to 206Po82,
in a notation where superscript before the element’s symbol represents the
atomic mass and the sub-script after the element’s symbol represents the atomic
number. The alpha particles, being heavy, are ejected at about 1/10th the speed
of light and are not very penetrating. They can travel about 1–4 inches in the
air.
2. Beta radiation is produced through beta decay of unstable
nuclei and can follow either of the three processes: electron emission,
positron emission, and electron capture.
·
Negative beta decay involves the emission of an energetic
electron and an antineutrino (which does not have a resting mass). In the
resulting nucleus, a neutron becomes a proton and stays in the nucleus. Thus,
the proton number (atomic number) of the result-ing nucleus increases by one,
while the mass number (total num-ber of protons and neutrons in the nucleus)
does not change. For example, this process occurs for tritium (3H) decay to
radioactive helium (3He).
·
Positive beta decay involves the emission of a positron,
similar to an electron in all aspects but with opposite charge, and a
neu-trino. In the resulting nucleus, a proton converts to a neutron. Thus, the
atomic number of the daughter nucleus is one less than the parent, while the
atomic mass remains the same.
·
Electron capture is a process whereby an orbiting electron
com-bines with a nuclear proton to form a neutron (which remains in the
nucleus) and a neutrino (which is emitted). In the resulting nucleus, the
atomic number reduces by one, while the atomic mass stays the same.
·
Beta decay is usually a slower process compared with alpha
or gamma decay. Most beta particles are emitted at the speed of light.
3. Gamma rays are the most penetrating electromagnetic
radiation of shortest wavelength and highest energy, just above the X-ray
region of the electromagnetic spectrum. Gamma rays can be produced by the decay
of the radioactive nuclei or of certain subatomic particles. The mechanism of
formation of high-energy gamma ray photons is currently not well understood.
Characteristic features of these types of radiations emitted by the
radioisotopes are summarized in Table 13.1.
Units of radioactivity
The
quantity of radioactive material is measured in terms of activity rather than
mass. The amount of radioactivity is typically expressed in the units of Curie
(Ci), which is a measure of radioactivity per unit mass of material. The
international system of units (SI system) recommends becquerel (Bq) as a unit
of radioactivity. One Bq represents the amount of radiation produced from one
disintegration per second (dps). One Ci is 37-billion Bq or 37 GBq.
While
Ci is the unit of measurement of radioactivity, the absorbed dose of ionizing
radiation is expressed in rad, the dose equivalent (when radiation is applied
to humans) is expressed in rem, and the exposure to radiation is quantitated in
roentgen (R). One rad represents the
amount of radiation that releases energy of 100 ergs per gram of matter. Erg is
a unit of energy or work that equals 10−7 Joules. Rem is the dosage
in rads that causes the same amount of biological injury as 1 rad of X-rays or
gamma rays.
Table 13.1 Characteristic features of different types of radiation emitted by
radioisotopes
In
the SI system of units, where Bq is the unit of radioactivity, gray (Gy) is the
unit of expression of absorbed dose, Sievert (Sv) is the dose equivalent unit,
and exposure is expressed in coulomb per kilogram body weight (C/kg). One rad
is 0.01 Gy and one rem is 0.01 Sv.
Radiation safety
Radiation
exposure can lead to several side effects that can be understood as the impact
of radiation on rapidly dividing cells. The following side effects are commonly
observed in patients undergoing radiation therapy of cancer:
·
Hair loss
·
Gastrointestinal irritation becoming evident as nausea,
vomiting, diarrhea, and stomach upset
·
Low white blood cell count (leucopenia).
·
Local side effects such as reddening and itchiness of the
skin, if applied
·
Oral mucositis, leading to sore mouth or oral ulcers
Generally,
doses higher than 30 μCi
are administered in a hospital setting to ensure adequate safety monitoring.
Avoiding
unintended exposure to radiation in a laboratory setting is a key function of
the organizational environmental, health, and safety (EHS) organizations. These
are done through careful inventory control, engineer-ing controls when handling
radioactive materials (such as the use of fume hoods), and proper storage and
disposal of radioactive material and con-taminated waste. In addition, the
following protection guidelines are rec-ommended for the users:
1. Time: The shorter the
time of potential use of a radioactive material, the shorter the duration of
exposure. Thus, quick and efficient work with minimal time of exposure of the
radioactive material to the ambient laboratory environment is recommended.
2. Distance: The farther a
person is from a source of radiation, the lower the dose of radiation exposure. In addition, physical contact with
the radioactive material is generally avoided with the use of devices to
manipulate or move stored containers of radioactive material.
3. Shielding: Radioisotopes are
typically handled in lead containers, since
lead absorbs and is impervious to all radiation. X-ray technicians and
laboratory personnel wear lead-coated aprons to block potential direct exposure
to radiation.
4. Quantity: The amount of
radioactive material in the working area and
inventory is generally minimized. Multiple procurements of small quantities are
preferred over purchasing and storing one large quantity.
Note
that temperature and pressure are not included in the list of radiation safety
considerations. In other words, handling a radioactive compound under
refrigerated conditions does not provide any lower exposure to radi-ation than
handling the same compound at the room temperature. The decay rate of
radionuclides is insensitive to temperature and pressure under normal usual
laboratory operating conditions.
Related Topics
