Proteins
| Home | | Anatomy and Physiology | | Anatomy and Physiology Health Education (APHE) |Chapter: Anatomy and Physiology for Health Professionals: Levels of Organization : Chemical Basics of Life
1. List some of the functions of proteins in the human body. 2. Explain how a peptide bond forms. 3. Differentiate between fibrous and globular proteins.
Proteins
Proteins are the most abundant organic componentsof the human body
and in many ways the most import-ant. They make up between 10% and 30% of cell
mass and are the basic structural materials of the body. Pro-teins are vital
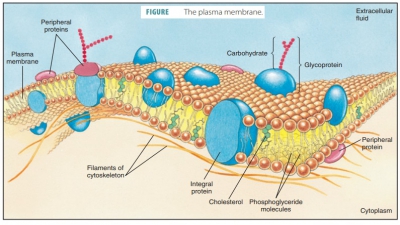
for many body functions. On cell sur-faces, some proteins combine with
carbohydrates to become glycoproteins.
They allow cells to respond to certain molecules that bind to them. Proteins
include biologic catalysts (enzymes), contractile proteins of muscles, and the
hemoglobin of the blood.
There are more than 200,000 types
of proteins in the human body, the full set known as the proteome.
Antibodies are proteins that detect and destroy foreign substances. All
proteins contain carbon, hydrogen, oxygen, and nitrogen atoms, with small
quantities of sulfur also present. Proteins always con-tain nitrogen atoms.
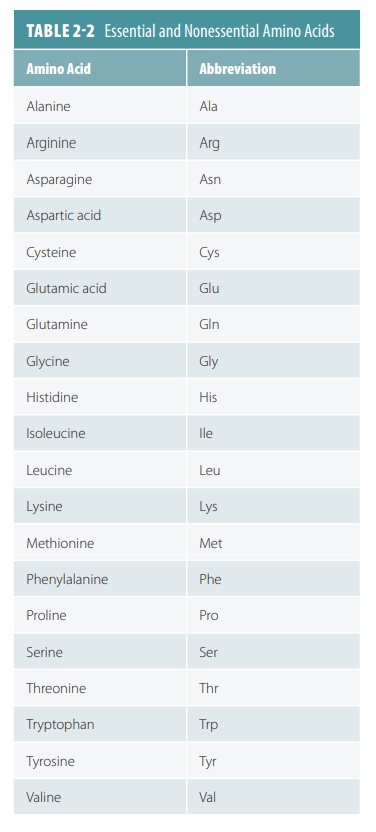
Twenty common amino acids, both essential and nonessential, make up the proteins that exist in
humans and most other living organ-isms (TABLE
2-2).
Amino acids are the building
blocks of proteins, with two primary groups: amines and organic acids.
Amino acids act as either bases (protonacceptors) or acids (proton donors). All
amino acids are exactly the same except for one group of atoms, known as the
amino acid’sR group. Differences in
the R group determine the chemical uniqueness of each amino acid (FIGURE 2-12).
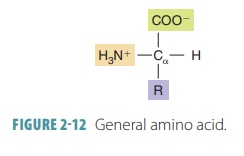
Protein molecules consisting of
amino acids held together by peptide bonds are called peptides, which are joined together via dehydration synthesis. The
amine end of one amino acid is linked to the acid end of the next amino acid.
This forms the characteristic atomic arrangement of a peptide bond. Each
type of peptide is named for the amount of amino acids that are united: dipeptide (2), tripeptide (3), polypeptide
(10 or more), and so on. Although most proteins are macromolecules, polypeptides that contain morethan 50 amino acids are
called proteins. Macromole-culesare
large and complex, with as few as 100 to over10,000 amino acids.
Every type of amino acid has its
own distinct properties. The way they bind determines how the proteins they
produce are structured and how they function. A change in one amino acid that
is linked to others produces an entirely unique function. Such a change can
also make the protein become nonfunc-tional. Examples of proteins include
insulin, oxytocin, and glucagon (FIGURE 2-13).
Types of Proteins
Proteins are generally classified
as either fibrous or globular. Fibrous
proteins are longer and resemble
“strands” and are highly stable and insoluble in water. They provide mechanical
support and tensile strength for body tissues. Collagen, the most abundant protein in the body, is a fibrous protein as are
elastin, keratin,and some contractile proteins found in muscles. Because of
their supporting functions, they are also called structural proteins.
Globular proteins are more compact than fibrousproteins and spherical in shape. They are chemically active and water-soluble. Globular proteins are import-ant in almost all biologic processes and are therefore also referred to as functional proteins. Examples of globular proteins are antibodies, protein-based hor-mones, and enzymes. Antibodies function in immunity, whereas protein-based hormones control growth and development. Enzymes are catalysts for nearly every chemical reaction taking place in the body.
1. List
some of the functions of proteins in the human body.
2. Explain
how a peptide bond forms.
3. Differentiate
between fibrous and globular proteins.