Plasma Lipoproteins
| Home | | Biochemistry |Chapter: Biochemistry : Cholesterol, Lipoprotein, and Steroid Metabolism
The plasma lipoproteins are spherical macromolecular complexes of lipids and specific proteins (apolipoproteins).
PLASMA LIPOPROTEINS
The plasma lipoproteins
are spherical macromolecular complexes of lipids and specific proteins
(apolipoproteins). The lipoprotein particles include chylomicrons,
very-low-density lipoproteins (VLDLs), low-density lipoproteins (LDLs), and
high-density lipoproteins (HDLs). They differ in lipid and protein composition,
size, density (Figure 18.13), and site of origin. [Note: Because lipoprotein
particles constantly interchange lipids and apolipoproteins with each other,
the actual apolipoprotein and lipid content of each class of particles is
somewhat variable.] Lipoproteins function both to keep their component lipids
soluble as they transport them in the plasma and to provide an efficient
mechanism for transporting their lipid contents to (and from) the tissues. In
humans, the transport system is less perfect than in other animals and, as a
result, humans experience a gradual deposition of lipid (especially
cholesterol) in tissues. This is a potentially life-threatening occurrence when
the lipid deposition contributes to plaque formation, causing the narrowing of
blood vessels (atherosclerosis).
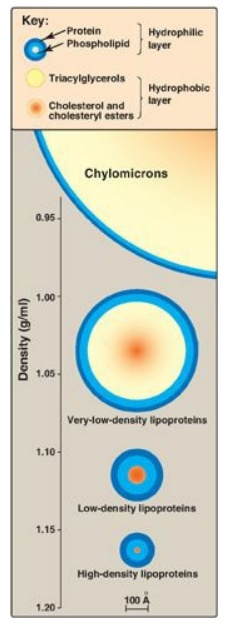
Figure 18.13 Approximate size and density of serum lipoproteins. Each family of lipoproteins exhibits a range of sizes and densities, and this figure shows typical values. The width of the rings approximates the amount of each component. [Note: Although cholesterol and its esters are shown as one component in the center of each particle, physically, cholesterol is a surface component, whereas cholesteryl esters are located in the interior of the lipoproteins.]
A. Composition of plasma lipoproteins
Lipoproteins are composed of a neutral lipid core (containing triacylglycerol [TAG] and cholesteryl esters) surrounded by a shell of amphipathic apolipoproteins, phospholipid, and unesterified (free) cholesterol (Figure 18.14). These amphipathic compounds are oriented so that their polar portions are exposed on the surface of the lipoprotein, thereby rendering the particle soluble in aqueous solution. The TAG and cholesterol carried by the lipoproteins are obtained either from the diet (exogenous source) or from de novo synthesis (endogenous source). [Note: The cholesterol (C) content of plasma lipoproteins is now routinely measured in fasting blood. Total C = LDL – C + HDL – C + VLDL – C, where VLDL – C is calculated by dividing TAG by 5 because TAG represent 20% of the volume of VLDL. The goal value for total cholesterol is less than 200 mg/dl.]
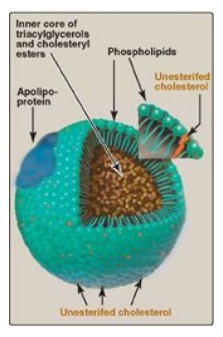
Figure 18.14 Structure of a typical lipoprotein particle.
1. Size and density of lipoprotein particles: Chylomicrons are the lipoprotein particles lowest in density and largest in size and that contain the highest percentage of lipid (as TAG) and the lowest percentage of protein. VLDLs and LDLs are successively denser, having higher ratios of protein to lipid. HDL particles are the smallest and densest. Plasma lipoproteins can be separated on the basis of their electrophoretic mobility, as shown in Figure 18.15, or on the basis of their density by ultracentrifugation.
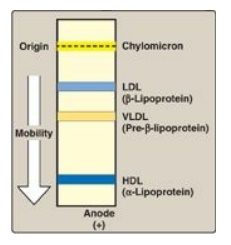
Figure 18.15 Electrophoretic mobility of plasma lipoproteins. The order of low-density lipoprotein (LDL) and very-low-density lipoprotein (VLDL) is reversed if ultracentrifugation is used as the separation technique. HDL = highdensity lipoprotein.
2. Apolipoproteins: The apolipoproteins associated with lipoprotein particles have a number of diverse functions, such as providing recognition sites for cell-surface receptors and serving as activators or coenzymes for enzymes involved in lipoprotein metabolism. Some of the apolipoproteins are required as essential structural components of the particles and cannot be removed (in fact, the particles cannot be produced without them), whereas others are transferred freely between lipoproteins. Apolipoproteins are divided by structure and function into several major classes, denoted by letters, with each class having subclasses (for example, apolipoprotein [apo] C-I, apo C-II, and apo C-III). [Note: Functions of all of the apolipoproteins are not yet known.]
B. Metabolism of chylomicrons
Chylomicrons are
assembled in intestinal mucosal cells and carry dietary (exogenous) TAG,
cholesterol, fat-soluble vitamins, and cholesteryl esters to the peripheral
tissues (Figure 18.16). [Note: TAGs account for close to 90% of the lipids in a
chylomicron.]
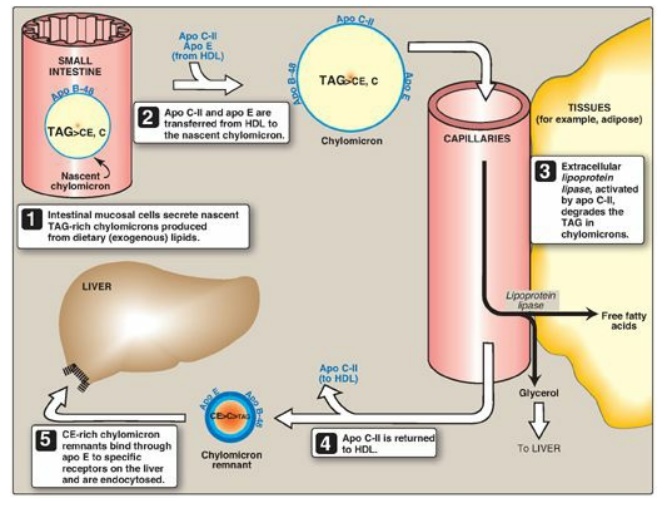
Figure 18.16 Metabolism of chylomicrons. Apo B-48, apo C-II, and apo E are apolipoproteins found as specific components of plasma lipoproteins. The lipoprotein particles are not drawn to scale (see Figure 18.13 for details of their size and density). TAG = triacylglycerol; C = cholesterol; CE = cholesteryl ester; HDL = high-density lipoprotein particle.
1. Synthesis of apolipoproteins: Apo B-48 is unique to
chylomicrons. Its synthesis begins on the rough ER, and it is glycosylated as
it moves through the ER and Golgi. [Note: Apo B-48 is so named because it
constitutes the N-terminal 48% of the protein encoded by the gene for apo B.
Apo B-100, which is synthesized by the liver and found in VLDL and LDL,
represents the entire protein encoded by this gene. Posttranscriptional editing
of a cytosine to a uracil in intestinal apo B-100 messenger RNA (mRNA) creates
a nonsense (stop) codon, allowing translation of only 48% of the mRNA.]
2. Assembly of chylomicrons: The enzymes involved in TAG, cholesterol, and phospholipid synthesis are located in the smooth ER. Assembly of the apolipoproteins and lipid into chylomicrons requires microsomal triglyceride transfer protein ([MTP]), which loads apo B-48 with lipid. This occurs before transition from the ER to the Golgi, where the particles are packaged in secretory vesicles. These fuse with the plasma membrane releasing the lipoproteins, which then enter the lymphatic system and, ultimately, the blood. [Note: Chylomicrons leave the lymphatic system via the thoracic duct that empties into the left subclavian vein.]
3. Modification of nascent chylomicron particles: The particle released by the intestinal
mucosal cell is called a “nascent” chylomicron because it is functionally
incomplete. When it reaches the plasma, the particle is rapidly modified,
receiving apolipoproteins E (which is recognized by hepatic receptors) and C.
The latter includes apo C-II, which is necessary for the activation of
lipoprotein lipase (LPL), the enzyme that degrades the TAG contained in the
chylomicron (see below). The source of these apolipoproteins is circulating HDL
(see Figure 18.16).
4. Degradation of triacylglycerol by lipoprotein lipase: LPL is an extracellular enzyme that is anchored by heparan sulfate to the capillary walls of most tissues, but predominantly those of adipose tissue and cardiac and skeletal muscle. Adult liver does not have this enzyme. [Note: A hepatic lipase is found on the surface of endothelial cells of the liver. It plays some role in TAG degradation in chylomirons and VLDL and is particularly important in HDL metabolism.] LPL, activated by apo C-II on circulating lipoprotein particles, hydrolyzes the TAG contained in these particles to yield fatty acids and glycerol. The fatty acids are stored (by the adipose) or used for energy (by the muscle). If they are not immediately taken up by a cell, the long-chain fatty acids are transported by serum albumin until their uptake does occur. The glycerol is used by the liver, for example, in lipid synthesis or gluconeogenesis. [Note: Patients with a deficiency of LPL or apo C-II (type 1 hyperlipoproteinemia, or familial LPL-deficiency) show a dramatic accumulation (1,000 mg/dl or greater) of chylomicron-TAG in the plasma (hypertriacylglycerolemia) even in the fasted state. These individuals are at increased risk for acute pancreatitis.]
5. Regulation of lipoprotein lipase activity: LPL is synthesized by adipose tissue and by cardiac and skeletal muscle. Expression of the tissue-specific isozymes is regulated by nutritional state and hormonal level. For example, in the fed state (elevated insulin levels), LPL synthesis is increased in adipose but decreased in muscle tissue. Fasting (decreased insulin) favors LPL synthesis in muscle. [Note: The highest concentration of LPL is in cardiac muscle, reflecting the use of fatty acids to provide much of the energy needed for cardiac function.]
6. Formation of chylomicron remnants: As the chylomicron circulates, and
more than 90% of the TAG in its core is degraded by LPL, the particle decreases
in size and increases in density. In addition, the C apolipoproteins (but not
apo E) are returned to HDL. The remaining particle, called a “remnant,” is
rapidly removed from the circulation by the liver, whose cell membranes contain
lipoprotein receptors that recognize apo E (see Figure 18.16). Chylomicron
remnants bind to these receptors and are taken into the hepatocytes by
endocytosis. The endocytosed vesicle then fuses with a lysosome, and the
apolipoproteins, cholesteryl esters, and other components of the remnant are
hydrolytically degraded, releasing amino acids, free cholesterol, and fatty
acids. The receptor is recycled. (A more detailed discussion of the mechanism
of receptor-mediated endocytosis is illustrated for LDL in Figure 18.20.)
C. Metabolism of very-low-density lipoproteins
VLDLs are produced in
the liver (Figure 18.17). They are composed predominantly of endogenous TAG
(approximately 60%), and their function is to carry this lipid from the liver
(site of synthesis) to the peripheral tissues. There, the TAG is degraded by
LPL, as discussed for chylomicrons. [Note: Nonalcoholic “fatty liver” (hepatic
steatosis) occurs in conditions in which there is an imbalance between hepatic
TAG synthesis and the secretion of VLDL. Such conditions include obesity and
type 2 diabetes mellitus.]
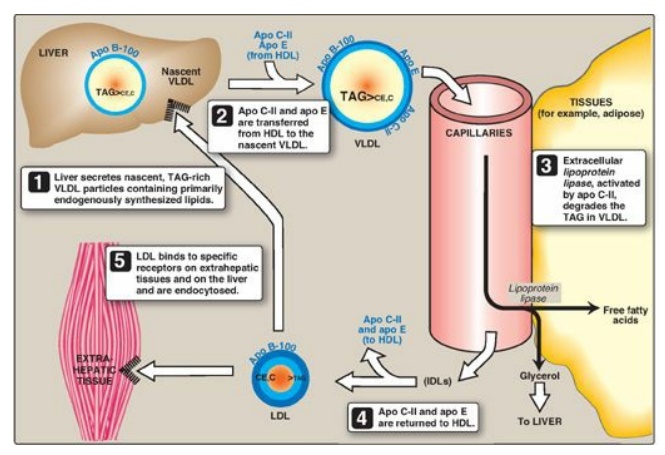
Figure 18.17 Metabolism of very-low-density lipoprotein (VLDL) and low-density lipoprotein (LDL). Apo B-100, apo C-II, and apo E are apolipoproteins found as specific components of plasma lipoprotein particles. The lipoproteins are not drawn to scale (see Figure 18.13 for details of their size and density). TAG = triacylglycerol; HDL = high-density lipoprotein particle; IDLs = intermediate-density lipoprotein particles; C = cholesterol; CE = cholesteryl ester.
1. Release from liver: VLDLs are secreted directly into
the blood by the liver as nascent particles containing apo B-100. They must
obtain apo C-II and apo E from circulating HDL (see Figure 18.17). As with
chylomicrons, apo C-II is required for activation of LPL. [Note:
Abetalipoproteinemia is a rare hypolipoproteinemia caused by a defect in MTP,
leading to an inability to load apo B with lipid. As a consequence, few VLDLs
or chylomicrons are formed, and TAGs accumulate in the liver and intestine.]
2. Modification in the circulation: As VLDLs pass through the
circulation, TAG is degraded by LPL, causing the VLDLs to decrease in size and
become denser. Surface components, including the C and E apolipoproteins, are
returned to HDL, but the particles retain apo B-100. Additionally, some TAGs
are transferred from VLDL to HDL in an exchange reaction that concomitantly
transfers cholesteryl esters from HDL to VLDL. This exchange is accomplished by
cholesteryl ester transfer protein (CETP) as shown in Figure 18.18.
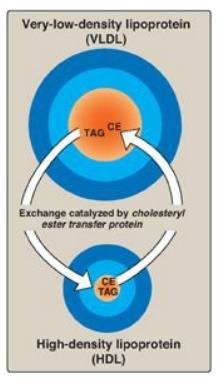
Figure 18.18 Transfer of cholesteryl ester (CE) from HDL to VLDL in exchange for triacylglycerol (TAG).
3. Conversion to low-density lipoproteins: With these modifications, the VLDL is converted in the plasma to LDL. Intermediate-sized particles, the intermediate-density lipoproteins (IDLs), or VLDL remnants, are observed during this transition. IDLs can also be taken up by liver cells through receptor-mediated endocytosis that uses apo E as the ligand. [Note: Apo E is normally present in three isoforms, E-2, (the least common), E-3 (the most common), and E-4. Apo E-2 binds poorly to receptors, and patients who are homozygotic for apo E-2 are deficient in the clearance of chylomicron remnants and IDLs. These individuals have familial type III hyperlipoproteinemia (familial dysbetalipoproteinemia, or broad beta disease), with hypercholesterolemia and premature atherosclerosis. Not as yet well understood is the fact that the E-4 isoform confers increased susceptibility to and decreased age of onset of the late-onset form of Alzheimer disease. The effect is dose dependent, with homozygotes being at greatest risk. Estimates of the risk vary.]
D. Metabolism of low-density lipoproteins
LDL particles contain
much less TAG than their VLDL predecessors and have a high concentration of
cholesterol and cholesteryl esters (Figure 18.19).
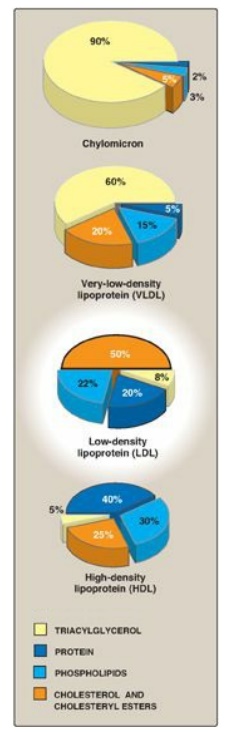
Figure 18.19 Composition of the plasma lipoproteins. Note the high concentration of cholesterol and cholesteryl esters in LDL.
1. Receptor-mediated endocytosis: The primary function of LDL
particles is to provide cholesterol to the peripheral tissues (or return it to
the liver). They do so by binding to cell surface membrane LDL receptors that
recognize apo B-100 (but not apo B-48). Because these LDL receptors can also
bind apo E, they are known as apo B-100/apo E receptors. A summary of the
uptake and degradation of LDL particles is presented in Figure 18.20. [Note:
The numbers in brackets below refer to corresponding numbers on that figure.] A
similar mechanism of receptor-mediated endocytosis is used for the cellular
uptake and degradation of chylomicron remnants and IDLs by the liver.
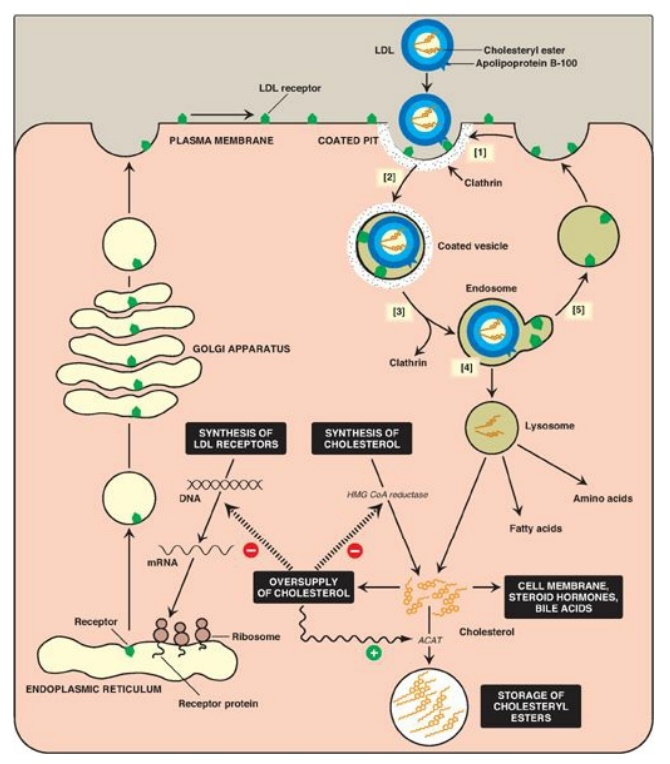
Figure 18.20 Cellular uptake and degradation of low-density lipoprotein (LDL). [Note: Oversupply of cholesterol accelerates the degradation of HMG CoA reductase. It also decreases synthesis of the reductase by preventing expression of its gene as seen with the LDL receptor.] ACAT = acyl CoA:cholesterol acyltransferase; HMG CoA = hydroxymethylglutaryl coenzyme A; mRNA = messenger RNA.
[1] LDL receptors are
negatively charged glycoproteins that are clustered in pits on cell membranes.
The cytosolic side of the pit is coated with the protein clathrin, which
stabilizes the pit.
[2] After binding, the
LDL–receptor complex is taken in by endocytosis. [Note: A deficiency of
functional LDL receptors causes a significant elevation in plasma LDL-C.
Patients with such deficiencies have type II hyperlipidemia (familial
hypercholesterolemia, or FH) and premature atherosclerosis. Autosomal-dominant
hypercholesterolemia can also be caused by increased activity of a protease, proprotein
convertase subtilisin/kexin type 9 (PCSK9), which promotes degradation of the
receptor, and by defects in apo B-100 that reduce its binding to the receptor.]
[3] The vesicle
containing LDL loses its clathrin coat and fuses with other similar vesicles,
forming larger vesicles called endosomes.
[4] The pH of the
endosome falls (due to the proton-pumping activity of endosomal ATPase), which
allows separation of the LDL from its receptor. The receptors then migrate to
one side of the endosome, whereas the LDLs stay free within the lumen of the
vesicle. [Note: This structure is called CURL, the compartment for uncoupling
of receptor and ligand.]
[5] The receptors can be recycled, whereas the lipoprotein remnants in the vesicle are transferred to lysosomes and degraded by lysosomal acid hydrolases, releasing free cholesterol, amino acids, fatty acids, and phospholipids. These compounds can be reutilized by the cell. [Note: Storage diseases caused by rare autosomal-recessive deficiencies in the ability to hydrolyze lysosomal cholesteryl esters (late-onset Wolman disease), or to transport free cholesterol out of the lysosome (Niemann-Pick disease, Type C) have been identified.]
2. Effect of endocytosed cholesterol on cellular cholesterol homeostasis: The chylomicron remnant-, IDL-, and LDL-derived cholesterol affects cellular cholesterol content in several ways (see Figure 18.20). First, expression of the gene for HMG CoA reductase is inhibited by high cholesterol, as a result of which, de novo cholesterol synthesis decreases. Additionally, degradation of the reductase is accelerated. Second, synthesis of new LDL receptor protein is reduced by decreasing the expression of the LDL receptor gene, thus limiting further entry of LDL cholesterol into cells. [Note: Regulation of the LDL receptor gene involves a SRE and SREBP-2, as was seen in the regulation of the gene for HMG CoA reductase. This allows coordinate regulation of the expression of these proteins.] Third, if the cholesterol is not required immediately for some structural or synthetic purpose, it is esterified by acyl CoA:cholesterol acyltransferase (ACAT). ACAT transfers a fatty acid from a fatty acyl CoA to cholesterol, producing a cholesteryl ester that can be stored in the cell (Figure 18.21). The activity of ACAT is enhanced in the presence of increased intracellular cholesterol.
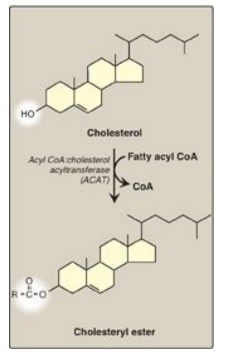
Figure 18.21 Synthesis of intracellular cholesteryl ester by ACAT. [Note: Lecithin:cholesterol acyl transferase (LCAT) is the extracellular enzyme that esterifies cholesterol using phosphatidylcholine (lecithin) as the source of the fatty acid.] CoA = coenzyme A.
3. Uptake of chemically modified LDL by macrophage scavenger receptors: In addition to the highly specific and regulated receptor-mediated pathway for LDL uptake described above, macrophages possess high levels of scavenger receptor activity. These receptors, known as scavenger receptor class A (SR-A), can bind a broad range of ligands and mediate the endocytosis of chemically modified LDL in which the lipid components or apo B have been oxidized. Unlike the LDL receptor, the scavenger receptor is not downregulated in response to increased intracellular cholesterol. Cholesteryl esters accumulate in macrophages and cause their transformation into “foam” cells, which participate in the formation of atherosclerotic plaque (Figure 18.22).
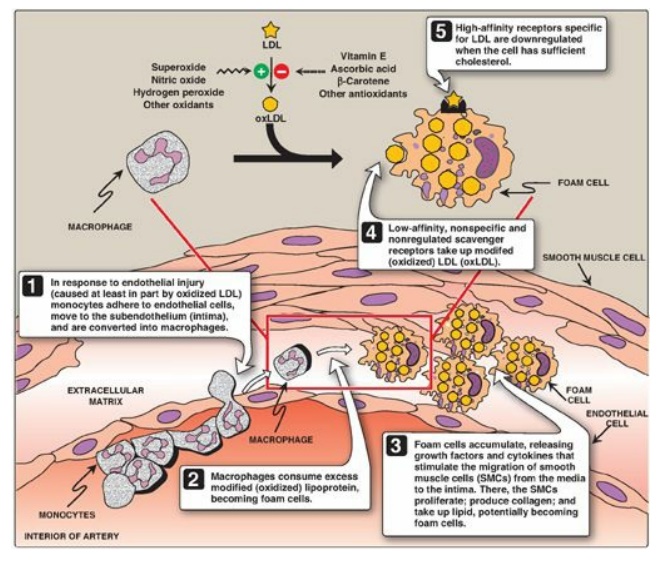
Figure 18.22 Role of oxidized lipoproteins in plaque formation in an arterial wall. LDL = low-density lipoprotein.
E. Metabolism of high-density lipoproteins
HDLs comprise a
heterogeneous family of lipoproteins with a complex metabolism that is not yet
completely understood. HDL particles are formed in blood by the addition of lipid
to apo A-1, an apolipoprotein made by the liver and intestine and secreted into
blood. [Note: HDLs are also formed within the liver and intestine.] Apo A-1
accounts for about 70% of the apolipoproteins in HDL. HDLs perform a number of
important functions, including the following.
1. Apolipoprotein supply: HDL particles serve as a
circulating reservoir of apo C-II (the apolipoprotein that is transferred to
VLDL and chylomicrons and is an activator o f LPL) and apo E (the
apolipoprotein required for the receptor-mediated endocytosis of IDLs and
chylomicron remnants).
2. Uptake of unesterified cholesterol: Nascent HDLs are disc-shaped particles containing primarily phospholipid (largely phosphatidylcholine) and apolipoproteins A, C, and E. They take up cholesterol from nonhepatic (peripheral) tissues and return it to the liver as cholesteryl esters (Figure 18.23). [Note: HDL particles are excellent acceptors of unesterified cholesterol as a result of their high concentration of phospholipids, which are important solubilizers of cholesterol.]
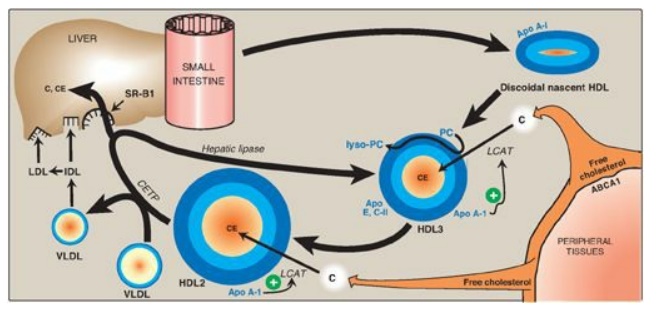
Figure 18.23 Metabolism of high-density lipoprotein (HDL) particles. Apo = apolipoprotein; ABCA1 = transport protein; C = cholesterol; CE = cholesteryl ester; LCAT = lecithin:cholesterol acyltransferase; VLDL = very-low-density lipoprotein; IDL = intermediate-density lipoprotein; LDL = low-density lipoprotein; CETP = cholesteryl ester transfer protein; SR-B1 = scavenger receptor B1.
3. Esterification of cholesterol: When cholesterol is taken up by HDL, it is immediately esterified by the plasma enzyme lecithin:cholesterol acyltransferase (LCAT, also known as PCAT, in which “P” stands for phosphatidylcholine, the source of the fatty acid). This enzyme is synthesized and secreted by the liver. LCAT binds to nascent HDL, and is activated by apo A-I. LCAT transfers the fatty acid from carbon 2 of phosphatidylcholine to cholesterol. This produces a hydrophobic cholesteryl ester, which is sequestered in the core of the HDL, and lysophosphatidylcholine, which binds to albumin. [Note: Esterification maintains the cholesterol concentration gradient, allowing continued efflux of cholesterol to HDL.] As the discoidal nascent HDL accumulates cholesteryl esters, it first becomes a spherical, relatively cholesteryl ester–poor HDL3 and, eventually, a cholesteryl ester–rich HDL2 particle that carries these esters to the liver. CETP moves some of the cholesteryl esters from HDL to VLDL in exchange for TAG, relieving product inhibition of LCAT. Because VLDLs are catabolized to LDL, the cholesteryl esters transferred by CETP are ultimately taken up by the liver.
4. Reverse cholesterol transport: The selective transfer of cholesterol from peripheral cells to HDL, from HDL to the liver for bile acid synthesis or disposal via the bile, and to steroidogenic cells for hormone synthesis, is a key component of cholesterol homeostasis. This process of reverse cholesterol transport is, in part, the basis for the inverse relationship seen between plasma HDL concentration and atherosclerosis and for HDL’s designation as the “good” cholesterol carrier. [Note: Exercise and estrogen raise HDL levels.] Reverse cholesterol transport involves efflux of cholesterol from peripheral cells to HDL, esterification of the cholesterol by LCAT, binding of the cholesteryl ester–rich HDL (HDL2) to liver (and steroidogenic cells), the selective transfer of the cholesteryl esters into these cells, and the release of lipid-depleted HDL (HDL3). The efflux of cholesterol from peripheral cells is mediated, at least in part, by the transport protein ABCA1. [Note: Tangier disease is a very rare deficiency of ABCA1 and is characterized by the virtual absence of HDL particles due to degradation of lipid-poor apo A-1.] The uptake of cholesteryl esters by the liver is mediated by a cell-surface receptor, SR-B1 (scavenger receptor class B type 1) that binds HDL. The HDL particle itself is not taken up. Instead, there is selective uptake of the cholesteryl ester from the HDL particle. [Note: Hepatic lipase, with its ability to degrade both TAG and phospholipids, also participates in the conversion of HDL2 to HDL3.]
ABCA1 is an ATP-binding cassette (ABC) protein. ABC
proteins use energy from ATP hydrolysis to transport materials, including
lipids, in and out of cells and across intracellular compartments. In addition
to Tangier disease, defects in specific ABC proteins result in sitosterolemia,
X-linked adrenoleukodystrophy, respiratory distress syndrome due to decreased
surfactant secretion, and cystic fibrosis.
F. Role of lipoprotein (a) in heart disease
Lipoprotein (a), or
Lp(a), is a particle that, when present in large quantities in the plasma, is
associated with an increased risk of coronary heart disease. Lp(a) is nearly
identical in structure to an LDL particle. Its distinguishing feature is the
presence of an additional apolipoprotein molecule, apo(a), that is covalently
linked at a single site to apo B-100. Circulating levels of Lp(a) are
determined primarily by genetics. However, factors such as diet may play some
role, as trans fatty acids have been shown to increase Lp(a), whereas ω-3 fatty
acids decrease it. [Note: Apo(a) is structurally homologous to plasminogen, the
precursor of a blood protease whose target is fibrin, the main protein
component of blood clots (See Chapter 34 online). It is hypothesized that
elevated Lp(a) slows the breakdown of blood clots that trigger heart attacks because
it competes with plasminogen for binding to fibrin. The physiologic function of
Lp(a) in unknown. Niacin reduces Lp(a), as well as LDL-cholesterol and TAGs,
and raises HDL.]
Related Topics
