Penicillins
| Home | | Pharmacology |Chapter: Essential pharmacology : Betalactam Antibiotics
Penicillin was the first antibiotic to be used clinically in 1941. It is a miracle that the least toxic drug of its kind was the first to be discovered. It was originally obtained from the fungus Penicillium notatum, but the present source is a high yielding mutant of P. chrysogenum.
PENICILLINS
Penicillin was the
first antibiotic to be used clinically in 1941. It is a miracle that the least
toxic drug of its kind was the first to be discovered. It was originally
obtained from the fungus Penicillium
notatum, but the present source is a high
yielding mutant of P. chrysogenum.
Chemistry And Properties
The penicillin nucleus
consists of fused thiazolidine and βlactam rings to which side chains are attached
through an amide linkage (Fig. 51.1). Penicillin G (PnG), having a benzyl side chain at R (benzyl
penicillin), is the original penicillin used clinically.
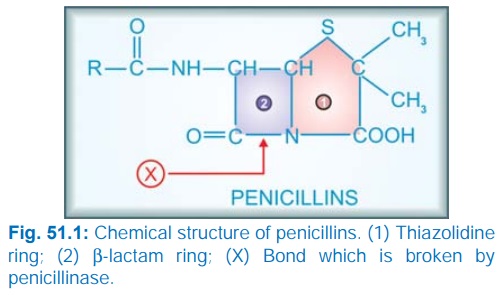
The side chain of
natural penicillin can be split off by an amidase to produce 6-aminopenicillanic
acid. Other side chains can then be attached to it resulting in different
semisynthetic penicillins with unique antibacterial activities and different
pharmacokinetic profiles.
At the carboxyl group
attached to the thiazolidine ring, salt formation occurs with Na+ and K+; these
salts are more stable than the parent acid. Sod. PnG is highly water soluble.
It is stable in the dry state, but solution deteriorates rapidly at room
temperature, though it remains stable at 4°C for 3 days. Therefore, PnG
solutions are always prepared freshly. PnG is also thermolabile and acid
labile.
Unitage 1 U of crystalline
sod. benzyl penicillin = 0.6 μg of the standard
preparation. Thus 1 g = 1.6 million units or 1 MU = 0.6 g.
Mechanism Of Action
All βlactam antibiotics interfere with the synthesis of bacterial
cell wall. The bacteria synthesize UDP-N-acetylmuramic acid pentapeptide,
called ‘Park nucleotide’ (because Park in 1957 found it to accumulate when susceptible
Staphylococcus was grown in the presence of
penicillin) and UDP-N-acetyl glucosamine. The peptidoglycan residues are linked
together forming long strands and UDP is split off. The final step is cleavage
of the terminal D-alanine of the peptide chains by transpeptidases; the energy
so released is utilized for establishment of cross linkages between peptide
chains of the neighbouring strands (Fig. 51.2). This cross linking provides
stability and rigidity to the cell wall.
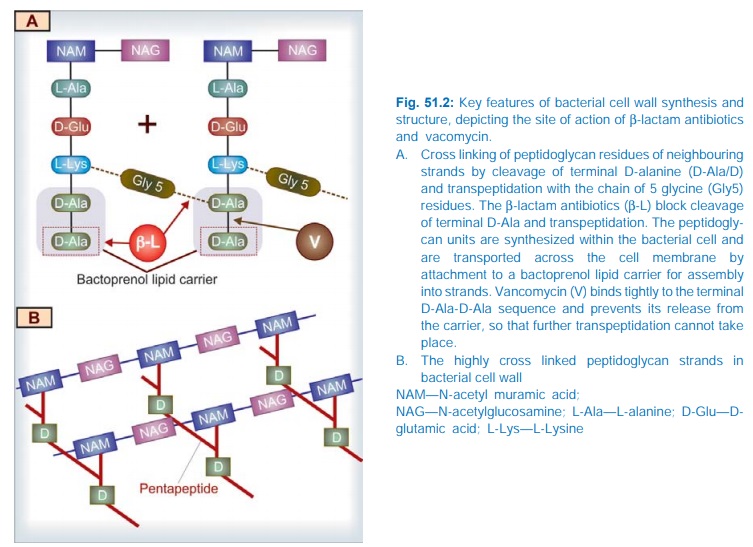
The βlactam antibiotics inhibit the transpeptidases so that cross
linking (which maintains the close knit structure of the cell wall) does not
take place. These enzymes and related proteins constitute the penicillin binding proteins (PBPs) which
have been located in the bacterial cell membrane. Each organism has several
PBPs and PBPs obtained from different species differ in their affinity towards
different βlactam antibiotics.
This fact probably explains their differing sensitivity to the various βlactam antibiotics.
When susceptible
bacteria divide in the presence of a βlactam antibiotic—cell wall deficient (CWD)
forms are produced. Because the interior of the bacterium is hyperosmotic, the
CWD forms swell and burst → bacterial lysis. This is how βlactam antibiotics
exert bactericidal action. Under certain conditions and in case of certain
organisms, bizarre shaped or filamentous forms, which are incapable of
multiplying, result. Grown in hyperosmotic medium, globular ‘giant’ forms or protoplasts are produced. Lytic effect
of these antibiotics may also be due to de-repression of some bacterial
autolysins which normally function during cell division.
Rapid cell wall synthesis occurs when the organisms are actively
multiplying; βlactam antibiotics are
more lethal in this phase.
The peptidoglycan cell wall is unique to bacteria. No such
substance is synthesized (particularly, D-alanine is not utilized) by higher
animals. This is why penicillin is practically nontoxic to man.
In gram-positive bacteria, the cell wall is almost entirely made
of peptidoglycan, which is >50 layers thick and extensively cross linked, so
that it may be regarded as a single giant mucopeptide molecule. In gram-negative
bacteria, it consists of alternating layers of lipoprotein and peptidoglycan
(each layer 1–2 molecule thick with little cross linking). This may be the
reason for higher susceptibility of the gram-positive bacteria to PnG.
Blood, pus, and tissue
fluids do not interfere with the antibacterial action of βlactam antibiotics.
