Oxidation States In Alkenes
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Oxidation States of Organic Compounds
For alkenes, several carbon oxidation levels are again possible. Furthermore, both carbon atoms must be considered as part of the same alkene functional group.
OXIDATION STATES IN ALKENES
For
alkenes, several carbon oxidation levels are again possible. Furthermore, both carbon atoms must be considered as
part of the same alkene functional group. While the total oxidation level can
go from −4 for ethylene (as
the sum of the oxidation level of both carbon atoms in the functional group) to
0 for a tetrasub-stituted alkene, we again recognize that all are of the same
functional class.
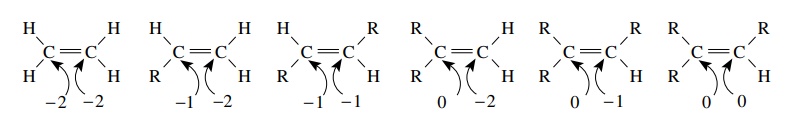
Furthermore
it is evident that because the lowest possible oxidation level of a single
carbon atom in an alkene is −2
while the lowest possible oxidation level of a carbon atom in an alkane is −4, alkenes are thus oxidized
relative to alkanes.
Related Topics
