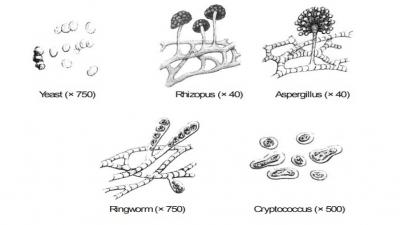
Nutrition, Cultivation and Isolation of Bacteria
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : Nutrition, Cultivation and Isolation of microorganisms : Bacteria-Actinomycetes-Fungi-Viruses
The nutrition, cultivation (growth), and isolation of bacteria shall be dealt with in the sections that follows :
BACTERIA
The
nutrition, cultivation (growth), and isolation of bacteria shall be dealt with
in the sections that follows :
1. Nutrition of Microorganisms (Bacteria)
Interestingly,
the microbial cell represents an
extremely complex entity, which is essentially comprised of approximately 70%
of by its weight as water, and the remaining 30% by its weight as the solid
components. Besides, the two major
gaseous constituents viz., oxygen (O2) and hydrogen (H2) the microbial cell predominantly consists
of four other major elements, namely :
Carbon (C), nitrogen (N), sulphur (S), and phosphorus (P). In fact, the
six aforesaid constituents almost account for 95% of the ensuing cellular dry
weight. The various other elements that also present but in relatively much
lesser quantum are : Na+, K+, Ca2+, Mg2+,
Mn2+, Co2+, Zn2+, Cu2+, Fe3+
and Mo4+. Based on these critical
observations and findings one may infer that the microorganisms significantly
require an excep-tionally large number of elements for its adequate survival as
well as growth (i.e., cultivation).
The
following Table 5.1 displays the various chemical composition of an Escherichia coli cell.
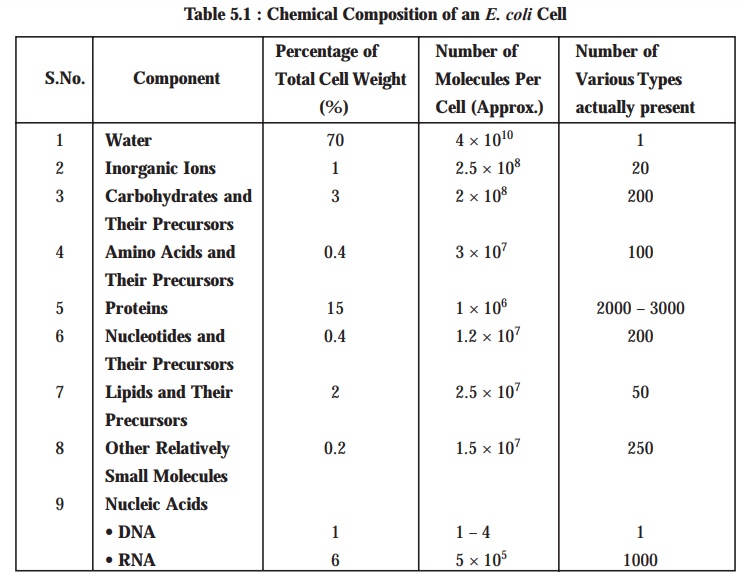
[Adapted From
: Tauro P et al. An Introduction to
Microbiology, New Age International, New Delhi, 2004]
It has
been amply proved and established that carbon
represents an integral component of almost all organic cell material ; and,
hence, constitutes practically half of the ensuing dry cell weight. Nitrogen is more or less largely
confined to the proteins, coenzymes, and the nucleic acids (DNA, RNA). Sulphur is a vital component of proteins and coenzymes ; whereas, phosphorus designates as the major
component of the nucleic acids.
It is,
however, pertinent to mention here that as to date it is not possible to
ascertain the precise requirement of various elements viz. C, N, S and O, by virtue of the fact that most bacteria
predomi-nantly differ with regard to the actual chemical form wherein these
elements are invariably consumed as nutrients.
2. Cultivation (Growth) of Bacteria
The cultivation (growth) of bacteria may be
defined, as — ‘a systematic progressive
increase in the cellular components’.
Nevertheless, an appreciable enhancement in
‘mass’ exclusively may not always
reflect the element of growth because bacteria at certain specific instances
may accumulate enough mass without a corresponding increment in the actual cell number. In the latest scenario the
terms ‘balanced growth’ has been
introduced which essentially draws a line between the so called ‘orderly growth’ and the ‘disorderly growth’.
Campbell
defined ‘balanced growth’ as — ‘the two-fold increase of each biochemical
unit of the cells very much within
the prevailing time period by a single division without having a slightest
change in the rate of growth’. However, one may accomplish theoretically
cultures with a ‘balanced growth’ having
a more or less stable and constant chemical composition, but it is rather next to impossible to achieve this.
Following
are some of the cardinal aspects of cultivation
of bacteria, such as :
Binary Fission
It has
been established beyond any reasonable doubt that the most abundantly available
means of bacterial cultivation (reproduction) is binary fission, that is, one
specific cell undergoes division to give rise to the formation of two cells.
Now, if
one may start the process with a single
bacterium, the corresponding enhancement in population is given by the following
geometric progression :
1 —→ 2 —→ 22 —→ 23 —→ 2′ —→ 25 —→ 26 —→ 2n
where, n = Number of generations.
Assuming
that there is no cell at all, each
succeding generation shall give rise to double
its death population. Thus, the
total at the end of a specific given time period may be expressed population ‘N’ as follows :
N = 1 × 2n ...(a)
Furthermore,
under normal experimental parameters, the actual number of organisms N0
inocu-lated at time ‘zero’ is not ‘1’ but most probably may range between
several thousands. In such a situa-tion, the aforesaid ‘formula’ may now be
given as follows :
N = N0
× 2n...(b)
Now,
solving Eqn. (b) for the value of ‘n’, we may have :
log10
N = log10 N0 + n
log10 2
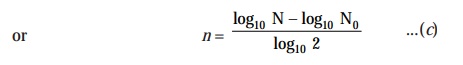
Substituting
the value of log10 2 (i.e.,
0.301) in Eqn. (c) above, we may ultimately
simplify the equation to :
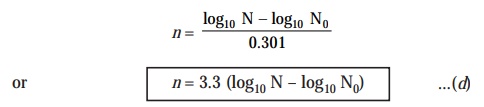
n = 3.3 (
log10 N - log10 N0 )
Application
of Eqn. (d), one may calculate quite
easily and conveniently the actual ‘number
of generations’ which have
virtually occurred, based on the precise data with respect to the following two experimental stages, namely :
(i) Initial
population of bacteria, and
(ii) Population
after growth affected.
Normal Growth Curve (or Growth Cycle) of Microorganisms :
Importantly,
one may describe the pattern of normal
growth curve (or growth cycle)
of micro-organisms by having an assumption that a ‘single microorganism’ after being carefully inoculated into a
sterilized flask of liquid culture medium aseptically which is incubated
subsequently for its apparent desired growth in due course of time. At this
point in time the very ‘seeded
bacterium’ would have a tendency to undergo ‘binary fission’ (see Section 2.2.1), thereby safely plunging into
an era of rapid growth and development whereby the bacterial cells shall undergo
‘multiplication in an exponential manner’. Thus, during the said span of rapid growth, if one takes
into consideration the theoretical
number of microorganisms that must be present at different intervals of
time, and finally plot the data thus
generated in the following two ways,
namely :
(a) Logarithm
of number of microorganisms, and
(b) Arithmatic
number of microorganisms Vs time.
one would
invariably obtain the ‘Curve’ as
depicted in Figure : 5.1.
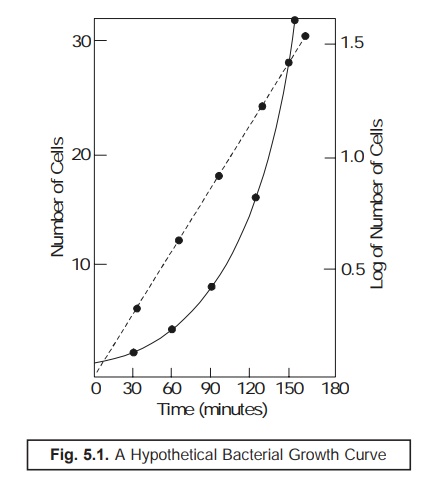
From Fig.
5.1 one may rightly derive the following three
valued and critical informations, such as :
·
Population gets increased regularly,
·
Polulation gets doubled at regular time intervals
(usually referred to as the ‘generation
time’) while under incubation, and
·
Exponential
growth designates only one particular segment of the ‘growth cycle’ of a population.
The Lag Phase of Microbial Growth
In actual
practice, however, when one carefully inoculates a fresh-sterilized culture
medium with a stipulated number of cells, subsequently finds out the ensuing bacterial population intermittently
under the following two experimental
parameters :
(a) during
an incubation period of 24 hours,
and
(b) plot
the curve between logarithms of the number
of available microbial cells Vs time (in minutes),
thereby
obtaining a typical curve as illustrated in Fig. 5.2.
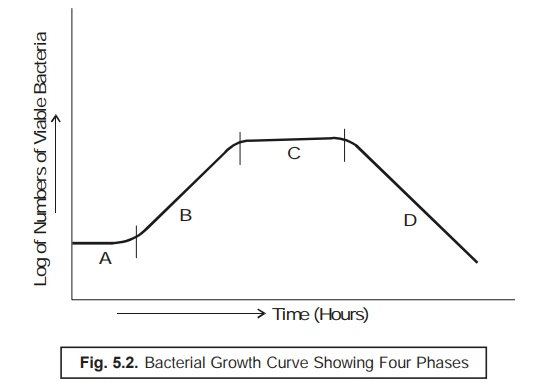
Curve A : Lag Phase ; Curve B : Exponential Phase
or Log (Logarithmic) Phase ;
Curve C : Stationary Phase ; and Curve D : Death
(or Decline) Phase.
From Fig.
5.2. one may distinctly observe the following salient features :
Lag Phase — i.e., at initial stages
there exist almost little growth of bacteria,
Exponential (or Log) Phase — i.e.,
showing a rather rapid growth,
Stationary Phase — i.e., depicting clearly
a levelling off growth of microbes, and
Death (or Decline) Phase — i.e.,
showing a clear cut decline in the viable population of microorganisms.
Translational Periods Between Various Growth Phases
A close
look at Fig. 5. 2 would reveal that a culture invariably proceeds rather slowly
from one particular phase of growth to the next phase. Therefore, it
categorically ascertains the fact that all the bacterial cells are definitely
not exposed to an identical physiological condition specifically as they
approach toward the end of a given phase of growth. Importantly, it involves
critically the ‘time factor’
essentially needed for certain bacteria to enable them catch up with the others
in a crowd of microbes.
Synchronous Growth
It has
been duly observed that there are quite a few vital aspects with regard to the internsive microbiological research wherein it might be possible to decepher
and hence relate the various aspects of
the bacterial growth, organization, and above all the precise differentiation
to a specific stage of the cell-division
cycle. However, it may not be practically feasible to carry out the
analysis of a single bacterium due
to its extremely small size. At this stage if one may assume that all the
available cells in a culture medium were supposed to be
having almost the same stage of the specific
division cycle, the ultimate result from the ensuing analysis of the cell
crop might be logically interpreted equivalent to a single cell. With the advent of several well elaborated and practised laboratory methodologies one could conveniently manipulate the on
going growth of cultures whereby all the available cells shall essentially be
in the same status of their ensuing
growth cycle. i.e., having a synchronus growth.
Salient Features : The
various salient features pertaining
to the aforesaid synchronous growth are
as stated under :
(1) Synchrony invariably lasts for a few generations, because even the daughters of a
single cell usually get out of phase
with one another very much within a short span.
(2) The
prevailing population may be synchronized judiciously by carrying out the
manipula-tion either of the chemical composition of the culture medium or by
altering the physical environment of the culture medium.
Example : The above hypothesis may be
expatiated by subjecting the bacterial
cells to a careful inoculation
into a culture medium duly maintained at a suboptimal
temperature. Interestingly, under these prevailing circumstances after a
certian lapse of time the bacterial cells shall metabolize gradu-ally, but certainly may not undergo cell division. However, when the temperature is
enhanced from the suboptimal level
to the elevated stage, the bacterial cells shall undergo a synchronized division.
(3) Interestingly,
the smallest microbial cells that are usually present in a specific log-phase culture do
happen to be those that have just divided ; and hence, lead to the most
abundantly known method of
synchronization. Besides, when these cells are duly subjected to separation either
by differential centrifugation or by
simple filtration, they are far
better syn-chronized with each other explicitely.
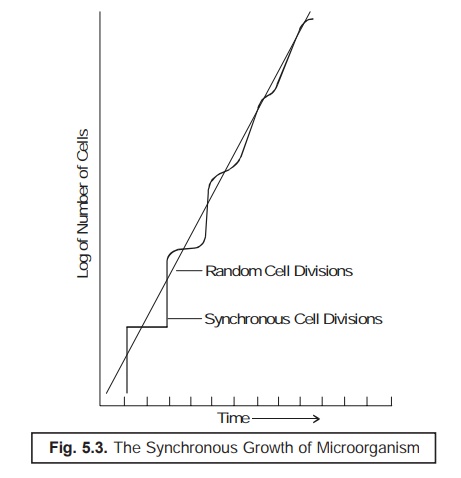
Fig. 5.3
illustrates the observed actual growth pattern of a definite population of the
available synchronized bacterial cells as given under.
The
steplike growth pattern, as depicted in Fig. 5.3 clearly shows that practically
all the cells of the population invariably undergo division at about the same
time.
Effect of Nutrient Concentration Vs Growth Rate of Bacterial Culture
In order
to have a comprehensive understanding with regard to the effect of the nutrient
concen-tration (substrate) upon the ensuing growth rate of the bacterial
culture one should duly take into consid-eration the existing relationship
between the exponential growth (R)
and the nutrient (substrate) concentration, which eventually does
not hold a simple linear relationship as
shown in Fig. 5.4.
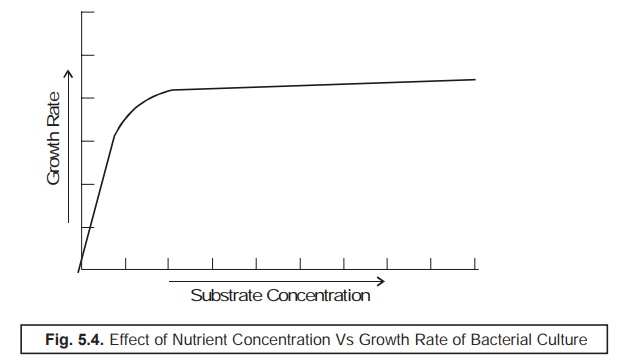
Growth Determining Techniques
As to
date there are several both direct and
indirect methodologies whereby one may accom-plish the following two cardinal aspects with respect to the
growth of microorganisms, namely :
(a) to
determine growth of bacteria, and
(b) to
determine growth rates of microorganisms.
In actual
practice, however, the ‘choice of the
method’ will exclusively depend upon whether the candidate organism is
either bacteria or fungi ; besides, several inherent
characteristic features of the microorganisms, for instance : clumping*.
Direct Method. It
essentially comprises of the following vital steps :
·
To determine precisely the enhancement of
the cell number,
·
Dry weight of bacteiral cell vis-a-vis
function of time (minutes/hours), and
·
Enhancement in any other cellular
component vis-a-vis function of time (minutes/hours).
Indirect
Method : It predominantly involves the inclusion of two
important ‘Optical Density’ measurements, such as : (/) optical
density, and (/'/') optical turbidity (using Nephelometer).
In short, the direct methods for the
determination of the ultimate growth by the aid of cell number are
invariably utilized with such organisms as : (a)
bacteria - that undergo binary fission ;
and (b) yeast -
that undergo the ‘budding’* phenomenon.
Summararily, the indirect
methods for the precise determination either bacteria or yeast may
be duly accomplished by the use of Turbidometers (for translucent
liquids), and Colorimeters (for transparent liquids), whereby
the observed density of the ensing cell suspension may be measured
accurately.
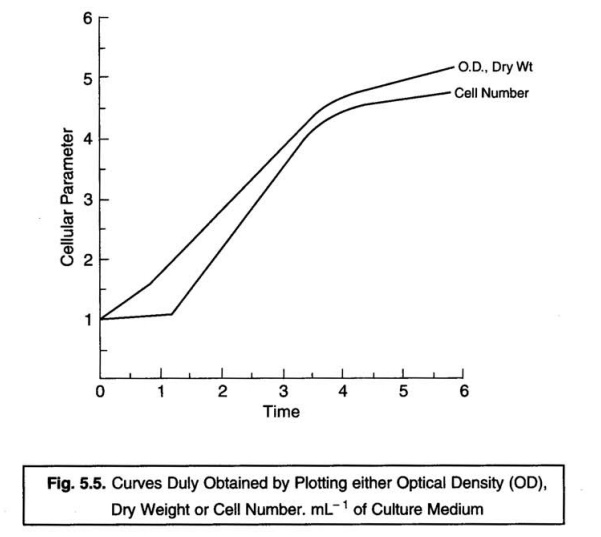
Fig. 5.5 illustrates the kind of curves which one obtains
when the ensuing growth is invariably measured in a liquid medium by various
methods. It has been amply proved and established that the actual changes which
take place in the cell population strategically after the inoculation into the
fresh growth medium are represented more accurately and precisely by the dry weight or optical density measurements.
3. Isolation of Bacteria
The
isolation of ‘Bacteria’ may be
accomplished in several recognized and well-established methods, such as :
(a) Selective
and diagnostic media,
(b) Bismuth
sulphite agar, and
(c) Selective
media for staphylococci.
The
aforesaid three methodologies
invariably used for the isolation of
bacteria shall be treated individually in the sections that follows :
(a) Selective and Diagnostic Media
McConkey’s
medium was first and foremost introduced in 1995 so as to isolate Enterobacteriaceae from faeces, urine,
foods, water etc. The medium essentially comprises of several nutrients viz., bile salts, lactose, and an appropriate indicator.
Bile salts categorically serve as an important natural surface-active agent which,
fails to inhibit the growth of the Enterobacteriaceae, but distinctly
inhibits the growth of the specific Gram-positive microorganisms that are
probably present in the material to be examined.
Lactose aids in the production of ‘acid’ from E. coli and A. aerogenes upon this culture medium thereby changing the colour of the
suitable indicator added ; besides, the said two microorganisms may also adsorb a certain amount of the
indicator that may eventually get duly precipitated around the growing cells.
Importantly, the bacteria
responsible for causing typhoid and paratyphoid fever, and bacillary dysentery
fail to ferment lactose ; and,
therefore, the resulting colonies produced duly by these organisms appear to be
absolutely transparent.
Modifications of McConkey’s Medium — are as
stated under :
(1) Synthetic
surface-active agent may replace the ‘Bile
Salts’,
(2) Selectivity
of McConkey’s medium may be enhanced
significantly by the incorporation of inhibitory
dyes e.g. crystal violet, neutral red.
In fact, these dyes particularly suppress the growth of Gram-positive microorganisms viz., Staphylococci.
(b). Bismuth Sulphite Agar
The
discovery of the bismuth sulphite agar
medium dates back to 1920s for the identification of Salmonella typhi in pharmaceutical
preparations, foods, faeces, urine, and water. It
essentially comprises of a ‘buffered
nutrient agar’ consisting of bismuth
sulphate, ferrous sulphate, and an indicator
brilliant green.
E. coli gets
inhibited at a concentration 0.0025% of brilliant green employed, whereas another organism Salmonella typhi shall grow predominantly. It has been observed
that bismuth sulphite does exert certain degree of inhibitory effect upon E. coli.
S. typhi, in the
presence of glucose, causes reduction of bismuth sulphite to the corresponding bismuth
sulphide (i.e., a black compound), thereby
ascertaining the fact that the investigative organism may generate H2S from the S-containing amino acids in
the medium, which in turn shall interact with FeSO4 to produce a
distinct black precipitate of FeS
(ferrous sulphide).
(c) Selective Media for Staphylococci
The presence
of the Staphylococci organisms in
various specimens viz., pharmaceutical prod-ucts, food items, and pathological specimens, may ultimately cause food poisoning as
well as serious systemic infections.
A few
typical examples of selective media for various organisms are as follows :
(i) Enterobacteria — a
surface active agent serves as the main-selector.
(ii) Staphylococci — NaCl
and LiCl serve as the main selectors. Besides, Staphylococci in general are found to be sufficiently tolerant of
NaCl concentrations upto an extent of 7.5%.
Related Topics