Newtonian flow
| Home | | Pharmaceutical Drugs and Dosage | | Pharmaceutical Industrial Management |Chapter: Pharmaceutical Drugs and Dosage: Rheology
Newton’s law of flow states that the application of stress on a liquid leads to flow in the direct proportion to the amount of stress applied.
Newtonian flow
Newton’s
law of flow states that the application of stress on a liquid leads to flow in
the direct proportion to the amount of stress applied. The constant that
relates the flow of a liquid to the applied stress is called viscosity, η. Thus,
t = η•D
where,
D is the rate of flow and t is the
applied stress.
Fluids
that obey Newton’s law of flow are referred to as Newtonian fluids, and
fluids that deviate from the linear stress–flow proportionality expressed by the Newton’s law are called
non-Newtonian fluids.
Viscosity
can also be expressed as the constant that relates the shearing stress (force
per unit area applied parallel to the direction of flow of liquid) and shear
rate (volume of fluid flow per unit area of the liquid). Thus,

where:
force
per unit area (F′/A)
required to bring about flow is called the shearing stress (F)
dv/dr
is the rate of shear representing the velocity of fluid movement per unit distance from the plane of shear
stress
The
units of F′/A
are dynes per cm2 or Pzascal. The units of dv/dr are (cm/sec)/cm =
sec−1. Thus, the Standard International (SI) unit of viscos-ity is
Pascal*second (Pa*s). The more commonly used unit of viscosity is dyne*second/cm2,
which is named Poise (P).
10
Poise = 1 Pascal*second
10
centiPoise = 1 Poise
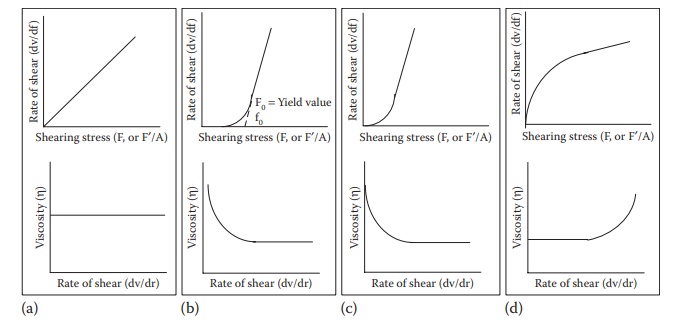
Figure 12.1 Plots of rate of shear and viscosity as a function of shearing stress
for (a) Newtonian,
(b) plastic, (c) pseudoplastic, and (d) dilatant flows.
The
higher the viscosity of a liquid, the greater the shearing stress required to
produce a certain rate of shear. A plot of the rate of shear against shearing
stress yields a rheogram. A Newtonian
fluid will plot a straight line, with the slope of the line being η (Figure
12.1a).
Another
term, fluidity, ϕ, is defined as the
reciprocal of viscosity:
Φ = 1/η (12.2)
In
the case of Newtonian fluids, viscosity is a constant for a fluid at a given
temperature and pressure. It does not change with increasing shear rate. Water
and dilute solutions typically exhibit Newtonian flow properties.
Temperature dependence and viscosity of liquids
Viscosity
of a liquid generally decreases as the temperature is raised. Increased
Brownian motion, rapid movement of liquid molecules among each other, at higher
temperatures reduces energy expense from the shear-ing force in overcoming
intermolecular cohesion forces, leading to a greater proportion of energy
utilized in influencing the direction of flow. In other words, fluidity
increases with an increase in temperature.
The
relationship between viscosity and temperature can be represented by the
following equation, which is analogous to the Arrhenius equation of chemical
kinetics:
η =
AeEv /RT (12.3)
where:
η is the viscosity (centiPoise)
A is the constant,
depending on molecular weight and molar volume of liquid
Ev is the activation
energy required to initiate flow between molecules (cal/mol)
R is the gas constant (1.987 cal*K−1*mol−1)
T is the temperature
in Kelvin
This
equation predicts that the decrease in viscosity with an increase in
temperature is nonlinear and follows an exponential profile. Thus, from the
temperature of highest viscosity of the fluid, slight changes in tempera-ture
lead to much more change in fluid viscosity compared with the effect of
temperature on viscosity close to the lowest viscosity of a fluid. The
temperature–viscosity relationships are critical for routine handling of
liquids in pharmaceutical manufacturing under ambient temperature conditions
and handling of drug products by patients. For example, an injectable solution
stored in a refrigerator may have much higher viscosity and may be difficult to
inject than the same solution brought to room temperature before injection.
Related Topics
