Molecules and Compounds
| Home | | Anatomy and Physiology | | Anatomy and Physiology Health Education (APHE) |Chapter: Anatomy and Physiology for Health Professionals: Levels of Organization : Chemical Basics of Life
1. Define the terms molecule, compound, and mixture. 2. List examples of true solutions in the body. 3. Define the terms mole, molarity, and molecular weight.
Molecules and
Compounds
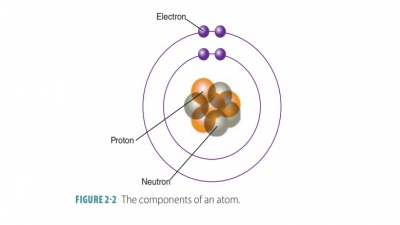
The term molecule is
defined as any chemical structure that consists of atoms held together by
covalent bonds (involving the sharing of electrons between atoms). When two
atoms of the same element bond, they produce molecules of that element such as
hydro-gen, oxygen, or nitrogen molecules. Most atoms are chemically combined
with other atoms. For example when two oxygen atoms combine, a molecule of O2
(oxygen gas) is formed.
When two different kinds of atoms
combine, they form molecules of a compound
(see “Covalent Bonds” later in this chapter). Compounds are chemicallypure, with identical molecules. A molecule is the
smallest particle of a compound that still has the spe-cific characteristics of
that compound. Examples of compounds include water (a compound of hydrogen and
oxygen), dry ice (frozen carbon dioxide), table sugar, baking soda, alcohol (as
used in beverages), natural gas, and most medicinal drugs. A molecule of a
compound has specific types and amounts of atoms (see “Hydrogen Bonds” later in
this chapter).
Mixtures
Mixtures are substances containing two or more com-ponents that are
physically intermixed. They do not
chemically combine and may not occur in fixed (spe-cific) proportions. An
example is the various powders combined together in a capsule with an active
drug. The individual characteristics of the components of a mixture are not
lost. They may be separated from each other if this is required. The three
basic types of mix-tures are colloids, solutions, and suspensions. In nature,
most matter exists in the form of mixtures. Colloids, solutions, and
suspensions are each found in both liv-ing and nonliving systems. Living
material is the most complex mixture of all, containing colloids, solutions,
and suspensions—all of which interact with each other.
Colloids
Colloids are also known asemulsions.
Their composi-tion differs in various areas of their mixtures, meaning they are
referred to as heterogeneous mixtures.
Colloids have solute particles that are larger than the particles in true
solutions and do not settle. The appearance of a colloid is often milky or
translucent. Light is scattered when shown through a colloid, meaning the path
of the light beam is visible.
Colloids often have the ability
to undergo sol–geltransformations.
This means they can change (revers-ibly) from a sol (fluid) state to a gel
(more solid) state. An example of a nonliving colloid that undergoes a sol–gel
transformation (when refrigerated) is a gelatin product such as Jell-O. Also,
the reverse process occurs when these products are heated, such as by sunlight,
with their state returning to a liquid. In living cells, the semifluid material
known as cytosol undergoes sol–gel transformations. Cytosol has many dispersed proteins, and these transformations are the basis
for cell division, cell shape changes, and other important activities.
Solutions
Solutions may be gases, liquids, or solids that arehomogeneous
mixtures of these components. This means the mixture has exactly the same
composition throughout in terms of the atoms
or molecules it contains. One sample of any part of the mixture will reveal an
identical composition to any other sample of the mixture. Examples include
ocean water (which is a mixture of water and salts) and environmental air
(which is a mixture of gases). In a mixture, the solvent is the substance that is present in the largest amount. A solvent is
also known as a dissolving medium and
is usually a liquid. The substances present in a mixture in smaller amounts are
called solutes.
In the human body, water is the
primary solvent. Most body solutions are called true solutions, which are usually transparent. They contain gases,
liquids, or solids dissolved in water. True solutions are exem-plified by
mineral water, glucose/water, and saline solution (which is a mixture of sodium
chloride and water). In true solutions, the solutes are minute, usu-ally
consisting of individual atoms and molecules. These microscopic solutes do not
scatter light (allow a beam of light to pass through) or settle.
True solutions are described by
their concentra-tion, which is often
described in parts per 100% of thesolute in the total solution. Water is
usually assumed to be the solvent. True solutions may also be described in milligrams per deciliter (mg/dL).
Another way to describe true solutions is molarity (M), which is defined as moles
per liter. This is a complicated but more chemically useful method. A mole is equal to an element or compound’s
atomic weight or molecularweight, in grams. For example, glucose is written asa combination
of 6 carbon atoms, 12 hydrogen atoms, and 6 oxygen atoms (C6H12O6).
To find its molecular weight, you must multiply the number of atoms of each
component by its atomic weight. Then, you must add the total atomic weights of
the three components to find the total atomic weight of glucose. In this
exam-ple, carbon’s 6 atoms multiplied by its atomic weight of 12.011 equals a
total atomic weight of 72.066. Using the same formula, the total atomic weights
of hydrogen (12.096) and oxygen (95.994) are added together to find that the
total atomic weight of glucose is 180.156. Overall, it is important to
understand that 1 mole of any substance always contains exactly the same
num-ber of solute particles. This is referred to as Avogadro’snumber, and is calculated in molecules of the substanceas
6.02 × 1023. In body fluids, because solute concentra-tions are very low, their
values are usually described in terms of millimoles
(Mmol or 1/1,000 mole).
Suspensions
Suspensions, known asheterogeneous
mixtures,have large, often visible solutes that usually settle. In the
blood, living blood cells are suspended
in the fluid portion of the blood (the blood
plasma). When a blood sample is left still for a period of time, the suspended
cells settle unless they are mixed or shaken. In the body, they do not settle
because of blood circulation.
1. Define
the terms molecule, compound, and mixture.
2. List
examples of true solutions in the body.
3. Define
the terms mole, molarity, and molecular weight.