Liver in Fasting
| Home | | Biochemistry |Chapter: Biochemistry : The Feed-Fast Cycle
The primary role of liver in energy metabolism during fasting is maintenance of blood glucose through the production of glucose from glycogenolysis and gluconeogenesis for glucose-dependent tissues and the synthesis and distribution of ketone bodies for use by other tissues.
LIVER IN FASTING
The primary role of
liver in energy metabolism during fasting is maintenance of blood glucose
through the production of glucose from glycogenolysis and gluconeogenesis for
glucose-dependent tissues and the synthesis and distribution of ketone bodies
for use by other tissues. Therefore, “hepatic” metabolism and “extrahepatic” or
“peripheral” metabolism are distinguished.
A. Carbohydrate metabolism
The liver first uses
glycogen degradation and then gluconeogenesis to maintain blood glucose levels
to sustain energy metabolism of the brain and other glucose-requiring tissues
in the fasted (postabsorptive) state. [Note: Recall that the presence of glucose
6-phosphatase in the liver allows the production of free glucose both from
glycogenolysis and from gluconeogenesis (see Figure 24.4).]
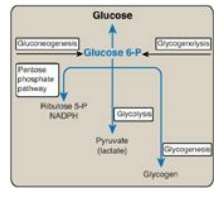
Figure 24.4 Central role of glucose 6-phosphate in metabolism. [Note: The presence of glucose 6-phosphatase in liver allows the production of free glucose from glycogenolysis and gluconeogenesis.] NADPH = nicotinamide adenine dinucleotide phosphate; P = phosphate.
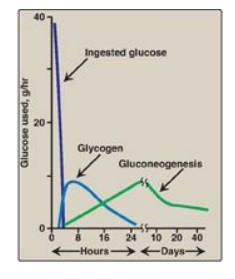
Figure 24.11 Sources of blood glucose after ingestion of 100 g of glucose. [Note: See Section B.2. for an explanation as to why gluconeogenesis declines.]
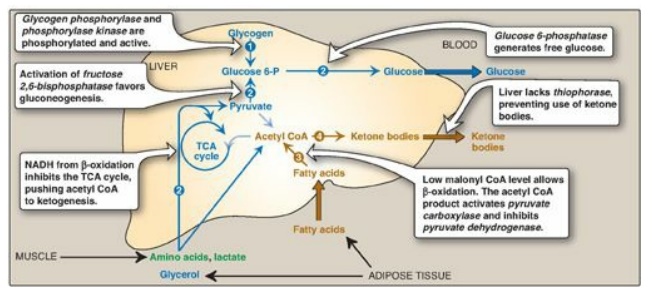
1. Increased glycogen degradation: Figure 24.11 shows the sources of blood glucose after ingestion of 100 g of glucose. During the brief absorptive period, ingested glucose is the major source of blood glucose. Several hours later, blood glucose levels have declined sufficiently to cause increased secretion of glucagon and decreased release of insulin. The increased glucagon-to-insulin ratio causes a rapid mobilization of liver glycogen stores (which contain about 80 g of glycogen in the fed state) due to PKA-mediated phosphorylation (and activation) of glycogen phosphorylase kinase that phosphorylates (and activates) glycogen phosphorylase. Figure 24.11 shows that liver glycogen is nearly exhausted after 10–18 hours of fasting, and therefore, hepatic glycogenolysis is a transient response to early fasting. Figure 24.12,1 , shows glycogen degradation as part of the overall metabolic response of the liver during fasting. [Note: Phosphorylation of glycogen synthase simultaneously inhibits glycogenesis.]
2. Increased glucose synthesis: The synthesis of glucose and its release into the circulation are vital hepatic functions during short- and long-term fasting (see Figure 24.12, 2 ). The carbon skeletons for gluconeogenesis are derived primarily from glucogenic amino acids and lactate from muscle and glycerol from adipose tissue. Gluconeogenesis, favored by activation of fructose 1,6-bisphosphatase (due to decreased availability of its inhibitor fructose 2,6-bisphosphate;) and by induction of PEPCK by glucagon, begins 4–6 hours after the last meal and becomes fully active as stores of liver glycogen are depleted (see Figure 24.11). [Note: The decrease in fructose 2,6-bisphosphate simultaneously inhibits glycolysis at PFK-1.]
B. Fat metabolism
1. Increased fatty acid oxidation: The oxidation of FAs obtained from TAG hydrolysis in adipose tissue is the major source of energy in hepatic tissue in the postabsorptive state (see Figure 24.12, 3). The fall in malonyl CoA due to phosphorylation (inactivation) of ACC by AMPK removes the brake on CPT-1, allowing β-oxidation to occur. FA oxidation generates NADH, FADH2, and acetyl CoA. The NADH inhibits the TCA cycle. The acetyl CoA is an allosteric activator of PC and an allosteric inhibitor of PDH, thereby favoring use of pyruvate in gluconeogenesis (see Figure 8.24). [Note: The acetyl CoA cannot be used as a substrate for gluconeogenesis, in part because the PDH reaction is irreversible.] Oxidation of NADH and FADH2 coupled with oxidative phosphorylation supplies the energy required by the PC and PEPCK reactions of gluconeogenesis.
2. Increased ketone body synthesis: The liver is unique in being able to synthesize and release ketone bodies, primarily 3-hydroxybutyrate but also acetoacetate, for use as fuel by peripheral tissues but not by the liver itself because liver lacks thiophorase. Ketogenesis, which starts during the first days of fasting (Figure 24.13), is favored when the concentration of acetyl CoA from FA oxidation exceeds the oxidative capacity of the TCA cycle. [Note: Ketogenesis releases CoA, ensuring its availability for continued FA oxidation.] The availability of circulating water-soluble ketone bodies is important in fasting because they can be used for fuel by most tissues, including brain, once their level in the blood is sufficiently high. This reduces the need for gluconeogenesis from amino acid carbon skeletons, thus preserving essential protein (see Figure 24.11). Ketogenesis as part of the overall hepatic response to fasting is shown in Figure 24.12, 4. [Note: Ketone bodies are organic acids and, when present at high concentrations, can cause ketoacidosis.]
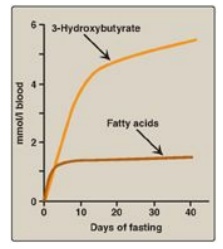
Figure 24.13 Concentrations of fatty acids and 3-hydroxybutyrate in the blood during fasting. [Note: 3-Hydroxybutyrate is made from the reduction of acetoacetate.]
Related Topics
