Levodopa
| Home | | Pharmacology |Chapter: Essential pharmacology : Antiparkinsonian Drugs
Levodopa has a specific salutary effect in PD: efficacy exceeding that of any other drug used alone. It is inactive by itself, but is the immediate precursor of the transmitter DA.
LEVODOPA
Levodopa has a specific salutary effect in PD: efficacy exceeding that of any other drug used alone. It is inactive by itself, but is the immediate precursor of the transmitter DA. More than 95% of an oral dose is decarboxylated in the peripheral tissues (mainly gut and liver). DA thus formed acts on heart, blood vessels, other peripheral organs and on CTZ (though located in the brain, i.e. floor of IV ventricle, it is not bound by blood-brain barrier). About 1–2% of administered levodopa crosses to the brain, is taken up by the surviving dopaminergic neurones, converted to DA which is stored and released as a transmitter. Brains of parkinsonian patients treated with levodopa till death had DA levels higher than those not so treated. Further, those patients who had responded well had higher DA levels than those who had responded poorly.
Actions
1. CNS
Levodopa hardly produces any effect in normal individuals or in patients with other neurological diseases. Marked symptomatic improvement occurs in parkinsonian patients. Hypokinesia and rigidity resolve first, later tremor as well. Secondary symptoms of posture, gait, handwriting, speech, facial expression, mood, self care and interest in life are gradually normalized.
The effect of levodopa on behaviour has been described as a ‘general alerting response’. In some patients this progresses to excitement— frank psychosis may occur. Embarrassingly disproportionate increase in sexual activity has also been noted. Dementia, if present, does not improve; rather it predisposes to emergence of psychiatric symptoms.
Levodopa has been used to produce a nonspecific ‘awakening’ effect in hepatic coma.
Two subtypes of DA receptors (D1, D2) were originally described. Three more (D3, D4, D5) have now been identified and cloned. All are G protein coupled receptors and are grouped into two families:
D1 like (D1, D5) Are excitatory: act by increasing cAMP formation and PIP2 hydrolysis thereby mobilizing intracellular Ca2+ and activating protein kinase C through IP3 and DAG.
D2 like (D2, D3, D4) Are inhibitory: act by inhibiting adenylyl cyclase/opening K+ channels/depressing voltage sensitive Ca2+ channels.
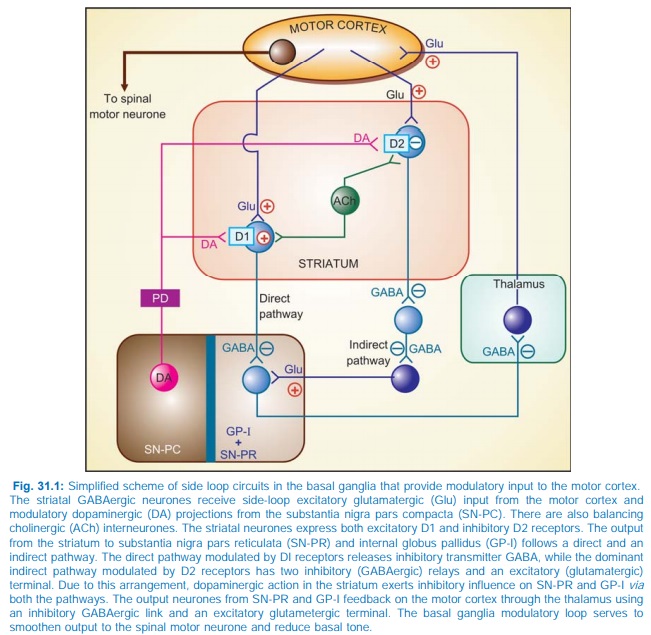
The various subtypes of DA receptors are differentially expressed in different areas of the brain, and appear to play distinct roles. Both D1 and D2 receptors are present in the striatum and are involved in the therapeutic response to levodopa. They respectively regulate the activity of two pathways having opposite effects on the thalamic input to the motor cortex (Fig. 31.1). Thus, stimulation of excitatory D1 as well as inhibitory D2 receptors in the striatum achieves the same net effect of smoothening movements and reducing muscle tone.
Dopamine receptor in SNPC and in pituitary is also of D2 type. The D3 receptors predominate in nucleus accumbans and hypothalamus, but are sparse in caudate and putamen, while D4 and D5 are mostly distributed in neocortex, midbrain, medulla and hippocampus.
2. CVS
The peripherally formed DA can cause tachycardia by acting on β adrenergic receptors. Though DA can stimulate vascular adrenergic receptors as well, rise in BP is not seen. Instead, postural hypotension is quite common. This may be a central action—DA and NA formed in the brain decrease sympathetic outflow; also DA formed in autonomic ganglia can impede ganglionic transmission.
Gradual tolerance develops to both cardiac stimulant and hypotensive actions.
3. CTZ
Dopaminergic receptors are present in this area and DA acts as an excitatory transmitter. The DA formed peripherally gains access to the CTZ without hindrance—elicits nausea
4. Endocrine
DA acts on pituitary mammotropes to inhibit prolactin release and on somatotropes to increase GH release. Though prolactin levels in blood fall during levodopa therapy, increased GH levels are not noted in parkinsonian patients. Probably the mechanisms regulating GH secretion are altered in these patients.
Pharmacokinetics
Levodopa is rapidly absorbed from the small intestines by utilizing the active transport process meant for aromatic amino acids. Bioavailability of levodopa is affected by:
1. Gastric emptying: if slow, levodopa is exposed to degrading enzymes present in gut wall and liver for a longer time—less is available to penetrate bloodbrain barrier.
2. Amino acids present in food compete for the same carrier for absorption: blood levels are lower when taken with meals.
Levodopa undergoes high first pass metabolism in g.i. mucosa and liver. The peripheral and central pathway of metabolism of levodopa is depicted in Fig. 31.2.
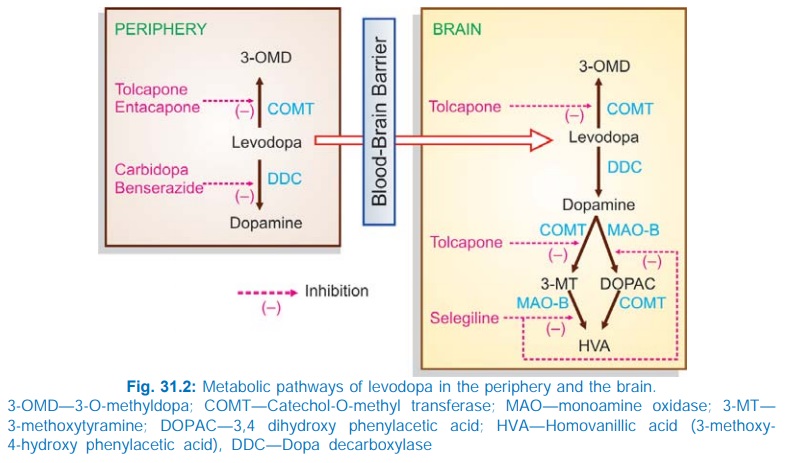
About 1% of administered levodopa that enters brain, aided by amino acid carrier mediated active transport across brain capillaries, also undergoes the same transformation. The plasma t½ of levodopa is 1–2 hours. Pyridoxal is a cofactor for the enzyme dopa-decarboxylase. The metabolites are excreted in urine mostly after conjugation.
Adverse Effects
Side effects of levodopa therapy are frequent and often troublesome. Most are dose-related and limit the dose that can be administered, but are usually reversible. Some are prominent in the beginning of therapy while others appear late.
At The Initiation Of Therapy
These can be minimized by starting with a low dose.
1. Nausea And Vomiting It occurs in almost every patient. Tolerance gradually develops and then the dose can be progressively increased.
2. Postural Hypotension It occurs in about 1/3 of patients, but is mostly asymptomatic; some patients experience dizziness, few have fainting attacks; more common in patients receiving antihypertensives. Tolerance develops with continued treatment and BP normalizes.
3. Cardiac Arrhythmias Due to β adrenergic action of peripherally formed DA; more in patients with pre existing heart disease.
4. Exacerbation Of Angina Due to β adrenergic action of peripherally formed DA; more in patients with pre existing heart disease.
5. Alteration In Taste Sensation
After Prolonged Therapy
1. Abnormal Movements Facial tics, grimacing, tongue thrusting, choreoathetoid movements of limbs start appearing after a few months of use of levodopa at optimum therapeutic dose and progress with time to include practically all patients. No tolerance develops to this adverse effect, but dose reduction decreases severity. Abnormal movements may become as disabling as the original disease itself—are the most important dose-limiting side effects.
2. Behavioral Effects Range from mild anxiety, nightmares, etc. to severe depression, mania, hallucinations, mental confusion or frank psychosis. Excessive DA action in the limbic system is probably responsible (antidopaminergic drugs are antipsychotic).
3. Fluctuation In Motor Performance After 2–5 years of therapy, the level of control of parkinsonian symptomatology starts showing fluctuation. ‘End of dose’ deterioration (wearing off) which is initially gradual, develops into rapid ‘switches’ or ‘onoff’ effect. With time ‘all or none’ response develops, i.e. the patient is alternately well and disabled. Abnormal movements may jeopardise even the ‘on’ phase. This is probably a reflection of progression of the disorder: with progressive degeneration of DA neurones the ability to regulate storage and release of DA may be largely lost: DA is then synthesized in the striatum on a moment to moment basis resulting in rapid and unpredictable fluctuations in motor control. Dose fractionation and more frequent administration tends to diminish these for a time.
Cautious use is needed in elderly; patients with ischaemic heart disease; cerebrovascular, psychiatric, hepatic and renal disease; peptic ulcer; glaucoma, gout.
Dose: Start with 0.25 g BD after meals, gradually increase till adequate response is obtained. Usual dose is 2–3 g/ day.
LEVOPA, BIDOPAL 0.5 g tab.
Interactions
Pyridoxine: Abolishes therapeutic effect by enhancing peripheral decarboxylation of levodopa; less is available to cross to the brain.
Phenothiazines, butyrophenones, metoclopramide reverse therapeutic effect of levodopa by blocking DA receptors. The antidopaminergic domperidone blocks levodopa induced nausea and vomiting without abolishing its antiparkinsonian effect, because domperidone does not cross bloodbrain barrier. Reserpine abolishes levodopa action by preventing entry of DA into synaptic vesicles.
Nonselective MAO inhibitors: prevent degradation of peripherally synthesized DA and NA—hypertensive crisis can occur.
Antihypertensives: postural hypotension is accentuated, reduce their dose if levodopa is started.
Atropine, and other anticholinergic drugs have additive antiparkinsonian action with low doses of levodopa, but retard its absorption— more time is available for peripheral degradation —efficacy of levodopa may be reduced.
