Isotope Effects
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Mechanisms of Organic Reactions
Besides the energy of the activated complex, structure and bonding in the acti-vated complex can be probed in several other ways using rate constant data.
ISOTOPE EFFECTS
Besides
the energy of the activated complex, structure and bonding in the acti-vated
complex can be probed in several other ways using rate constant data. One very
powerful way to investigate bonding in the activated complex is to use kinetic
isotope effects. Isotope effects derive from the fact that a heavier isotope of
an element has a lower zero-point energy and hence more energy is required to
break a bond to a heavier isotope than a bond to a lighter isotope (i.e., the
activation energy is greater). At a given temperature this means that the rate
of reaction for a compound containing a heavy isotope is slower than the rate
of reaction for the compound with a lighter isotope. This is only true if breaking of that bond is
involved at the transition state of the rate-determining step. If breaking of
this bond occurs prior to or after the rate-determining step, isotopic
substitution does not give a large change in the rate. This effect is most
pronounced for hydrogen/deuterium, which has the largest mass difference of any
isotopic pair and thus the largest difference in zero-point energies. If a bond
to hydrogen (or deuterium) is being broken in the rate-determining step, then kH/ kD values of
2 – 8 are typical. These are termed
primary kinetic deuterium isotope
effects.
If
the C–H(D) bond is not being broken in the rate-determining step, there are
sometimes smaller effects on the rate resulting from isotopic substitution that
are termed secondary kinetic
deuterium isotope effects. They result from zero-point energy differences in
deformation modes but they are small and typically kH/ kD values are 1 – 1.3 for these effects.
If a kinetic deuterium isotope effect is found
to be greater than about 1.5, it is a primary kinetic deuterium isotope effect
and C–H(D) bond breaking is occurring in the rate-determining step. If a
kinetic deuterium isotope effect is found to be between 1 and 1.5, it is a
secondary kinetic deuterium isotope effect and C–H(D) bond breaking is not
occurring in the rate-determining step.
The
largest values of primary kinetic deuterium isotope effects are found for
reactions where the bond to hydrogen is about one-half broken (kH/ kD values are 6 – 8). Smaller values are found in
reactions in which the bond to hydro-gen is less than or more than one-half
broken. Normally, kH/ kD values less than maximum
correspond to bond cleavage of < 1/2
.
Primary kinetic deuterium isotope
effects thus provide insight into the extent of C–H bond cleavage in the
activated complex.
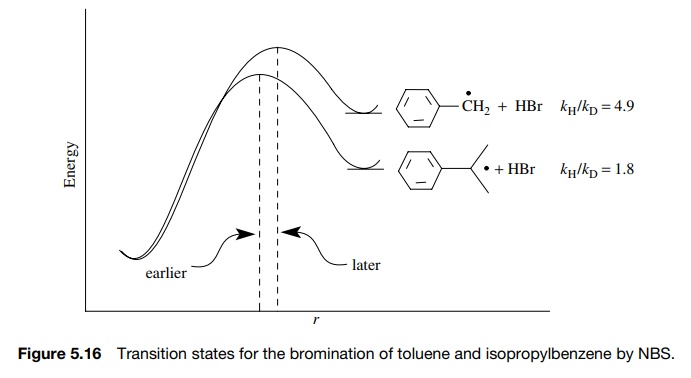
For
example, the free-radical bromination of toluene by N -bromosuccinimide (NBS) proceeds with kH/ kD
= 4.9, while for the same bromination of
isopropyl benzene, kH/ kD = 1.8 (Figure 5.16). Both are primary kinetic deuterium iso-tope
effects, indicating that hydrogen abstraction by a bromine atom is the
rate-determining step. The much lower value of the isotope effect for
isopropyl-benzene suggests that the transition state is much earlier than for
toluene. The lesser extent of hydrogen transfer in isopropylbenzene is due to
the more stable radical being produced, resulting in an earlier transition
state.
Therefore
The
electrophilic nitration of benzene using acetyl nitrate involves the
replace-ment of a hydrogen on the benzene ring by a nitro group. The reaction
is second order overall, first order in benzene, and first order in the
nitrating agent -ν = k[C6H6][acetyl
nitrate].
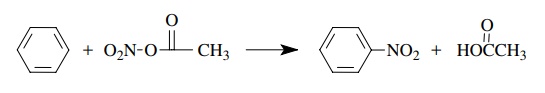
Use
of fully deuterated benzene gave kH/ kD = 1. These data suggest that the
nitrating agent attacks the benzene ring in the rate-determining step, but C–H
bond breaking is not involved in the rate-determining step. These observations
are consistent with an electrophilic attack of the nitrating agent of the π system. The proton is lost in a
subsequent fast step, after the rate-determining step.
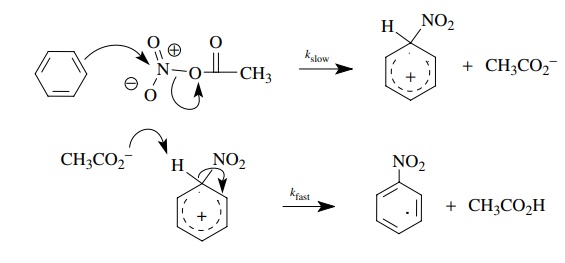
Thus,
even though loss of hydrogen is required for the product to be formed, its
removal is not taking place in the rate-determining step of the reaction — it
must take place after the rate-determining step.
The
base-promoted bromination of ketones is a second-order process, first order in
ketone and first order in base; thus ν
= k[ketone][base]. The bromine
concentration does not appear in the rate law; that is, the reaction is zero
order in [Br2].
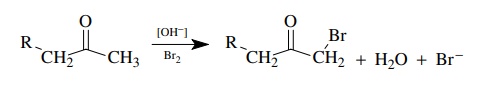
Use
of deuterated substrates gives kH/ kD = 6.5. This is a primary kinetic deu-terium isotope effect, indicating
that proton removal is an essential component of the rate-determining step. The
lack of rate dependence on bromine requires that bromine is added to the
molecule after the rate-determining step. A mechanism consistent with these
facts has proton removal and enolate formation rate determining.

If
we now take this basic scenario and add our notions of electron movement to the
picture, we can construct a detailed picture of electronic change that is
consistent with the observed facts.
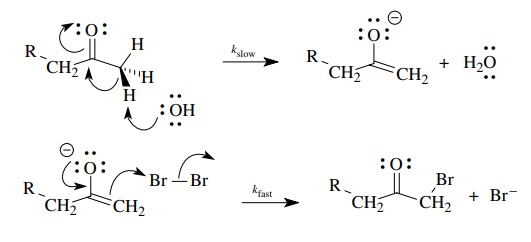
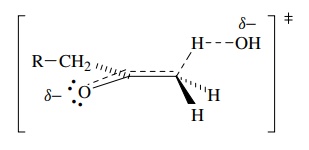
The
activated complex for proton removal, the rate-determining step, can be
envisioned as having a partial charge from proton removal delocalized into the
carbonyl group (as it is in the product enolate). This also requires that the
proton being removed has a dihedral angle of 90◦ with the plane of
the carbonyl group so that the developing charge can overlap with the carbonyl π bond.
Base-promoted
elimination in the two β-phenethyltrimethylammonium
deriva-tives shown below is found to be second order overall, first order in
substrate, and first order in base; that is, ν = k[C6H 5CH2CH2N+ (CH3)3][CH3CH2O−].
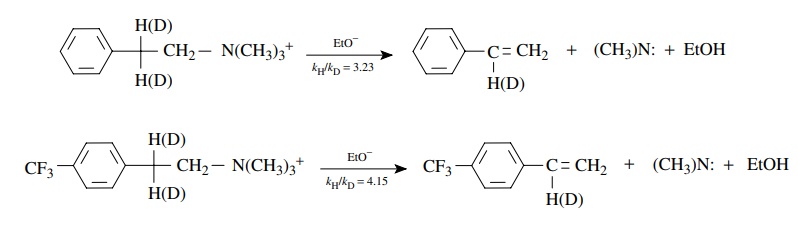
This
means that both the substrate and the ethoxide base are present in the
transition state of the rate-determining step. The rate constants for the
deuterated and protio substrates were measured. The magnitudes (kH/ kD =
3 – 4) of the kinetic deuterium
isotope effects for both substrates are typical primary kinetic deuterium
isotope effects, which means that C–H bond breaking is involved at the
transition state of the rate-determining step. This suggests that proton
removal by a base in the activated complex is an essential element of the
rate-determining step and is a key feature in the mechanism of the elimination
reaction.
The
difference between the kH/ kD values, however, means
that the extent of C–H bond breaking at the transition state in the second
substrate is different from the first. (The transition state of the second
substrate is actually earlier in terms of proton removal by the base because in
both cases proton transfer is greater than half completed.) Thus a change in
structure of the substrate leads to a distinct change in the structure of the
activated complex which can be detected and described by kinetic isotope
effects.
From
the above examples it is clear that kinetic deuterium isotope effects are a
powerful way to probe bonding changes in the activated complex. The magnitude
of the isotope effect indicates whether bonds to hydrogen are being made or
broken in the rate-determining step. Differences in kinetic isotope effects in
closely related precursors can also be used to pinpoint whether one transition
state is earlier than another — a direct measure of the effect of the substrate
structure on the structure of the transition state.
Other
elements can be used to measure isotope effects; however, the magni-tudes of
these isotope effects are much smaller than primary kinetic deuterium isotope
effects. Substitution of 13C for 12C in a reaction could
lead to a maximum kinetic isotope
effect of k12C/ k13C = 1.05 for a reaction in which a bond to carbon is broken in the
rate-determining step. (Recall that maximum kH/ kD’s are 8 – 10.) Most
standard kinetic methods are not capable of distinguishing such small rate
differences reproducibly, and so kinetic isotope effects for elements other
than hydrogen (deuterium) are not very abundant in the literature. In some
instances, isotopic abundances determined by mass spectrometry can be used to
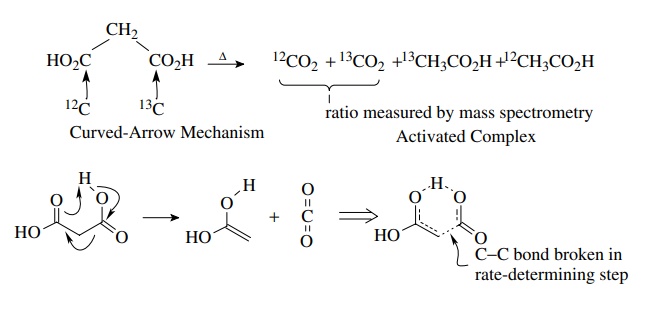
measure such differences accurately and isotope effects can be informative. The
decarboxylation of malonic acid proceeds with k12C/ k13C
= 1.045. This large primary-isotope effect
(for carbon) indicates that C–C bond breaking is well developed in the
transition state. This detailed information about the structure of the
activated complex permits a shift in focus from a curved-arrow type of
mechanism to a real structure of the activated complex.
Related Topics
