Ionization and pKa Value
| Home | | Medicinal Chemistry |Chapter: Medicinal Chemistry : Ionization and pKa Value
If the biological activity of a drug results from ions, the activity intensifies with increase in the degree of ionization.
Ionization and pKa Value
INTRODUCTION
If the
biological activity of a drug results from ions, the activity intensifies with
increase in the degree of ionization. However, if the activity results from
undissociated molecules, increase in the degree of ionization of active
compounds causes a decrease in activity.
Increase
in ionization intensifies a drug’s water solubility and decreases its
liposolubility. In general, drugs cross cellular membranes in undissociated
forms as intact molecules and act in dissociated forms as ions. This happens
because the passage of ions across the cellular membrane is prevented by two
factors.
1.
The
cellular membrane is made up of layers of electrically charged macromolecules
(lipids, proteins, and muco polysaccharide) that attract or repel ions.
2.
Hydration
of ions increases their volumes rendering difficult their diffusion through
pores.
Weakly
acidic drugs are predominantly of the unionized form at lower pH of the gastric
fluid, and absorbed from the stomach as well as intestine. Some very weak acidic
drugs, such as phenytoin and many barbiturates, whose pKa values are greater
than 7, are essentially unionized at all pH values. Therefore, for these weak
acidic drugs transport is more rapid and independent of pH.
Most weak bases are
poorly absorbed in the stomach since they are present largely in the ionized
form at low pH. Strong base, those with pKa values between 5 and 11, shows pH
dependent absorption. Stronger base, such as guanithidine (pKa > 11), are
ionized throughout the gastrointestinal tract and tend to be poorly absorbed.
pKa VALUE
The partially
lipidic nature of cellular membranes, such as the ones that enwrap the stomach,
small intestine, mucosa, and nervous tissue facilitate the passage of drugs
with high liposolubiltiy across them. The liposolubiltiy is affected by pH of
the environmental medium and by the degree of dissociation pKa. Usually, drugs
are weak acids or weak base. The degree of dissociation, pKa, is calculated
from the following Henderson–Hasselbalch equation.
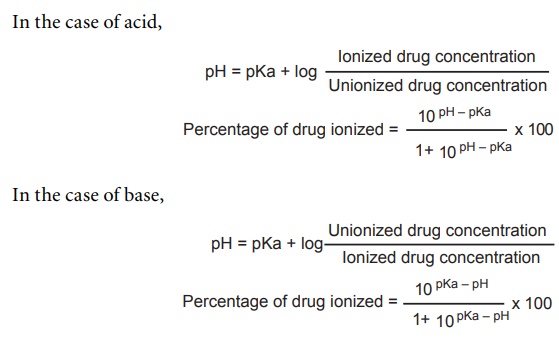
The
biological activity of certain acids and bases is directly related to their
degree of ionization. Whereas some (e.g. phenols, carboxylic acids) act in the
molecular form, others (quaternary ammonium salts) act in an ionized form. In these
cases, the pH plays an important role, that is, acids are more active at lower
pH; bases are more active at higher pH.
●
Strong
acid has low pKa value
●
Weak
acid has high pKa value
●
Strong
base has high pKa value
●
Weak
base has low pKa value
Drug Exerting Action as Undissociated Molecules
In a large number of
potent medical compounds, the dissociation plays a vital role for their
respective biological characteristics. The unusual structural grouping in the
tetracycline results in three distinct acidity constants in aqueous solutions
of the acid salts. The particular functional groups responsible for each of the
thermodynamic pKa value have been determined by Lessen et al, as described in
Figure 4.1.
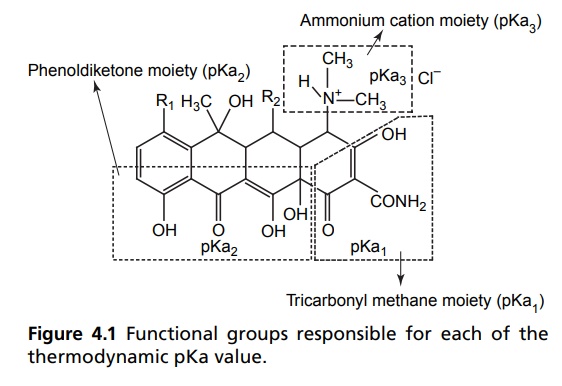
The approximate pKa
values for each of these groups in the four commonly used tetracyclines are
shown in Table 4.1.

Besides the activities of several local anaesthetics, d-tubocurarine and phenol have also been proved to be related to their degree of ionization.
Drug Exerting Action as Ionized Molecules
A plethora of
medicinal compound exerts their pharmacodynamic action exclusively as the
ionized molecule, namely, acetylcholine, quartenary salts as ganglionic
blocking agents, muscle relaxants, and antiseptics.
