Interfacial Mass Transfer
| Home | | Pharmaceutical Technology |Chapter: Pharmaceutical Engineering: Mass Transfer
At the interface, equilibrium conditions exist. The break in the curve is due to the different affinities of component A for the two phases and the different units expressing concentration.
INTERFACIAL MASS TRANSFER
So
far, only diffusion in the boundary layers of a single phase has been
dis-cussed. In practice, however, two phases are normally present and mass
transfer across the interface must occur. On a macroscopic scale, the interface
can be regarded as a discrete boundary. On the molecular scale, however, the
change from one phase to another takes place over several molecular diameters.
Because of the movement of molecules, this region is in a state of violent
change, the entire surface layer changing many times a second. Transfer of
molecules at the actual interface is, therefore, virtually instantaneous, and
the two phases are, at this point, in equilibrium.
Since
the interface offers no resistance, mass transfer between phases can be
regarded as the transfer of a component from one bulk phase to another through
two films in contact, each characterized by a mass transfer coefficient. This
is the two-film theory and is the simplest of the theories of interfacial mass
transfer. For the transfer of a component from a gas to a liquid, the theory is
described in Figure 4.3. Across the gas film, the concentration, expressed as
partial pressure, falls from a bulk concentration PAg to an
interfacial concentration PAi. In the liquid, the concentration
falls from an interfacial value CAi to a bulk value CAl.
At
the interface, equilibrium conditions exist. The break in the curve is due to
the different affinities of component A for the two phases and the different
units expressing concentration. The bulk phases are not, of course, at
equilib-rium, and it is the degree of displacement from equilibrium conditions
that provides the driving force for mass transfer. If these conditions are
known, an overall mass transfer coefficient can be calculated and used to
estimate the rate of mass transfer.
Transfer
of a component from one mixed phase to another, as described above, occurs in
several processes. Liquid-liquid extraction, leaching, gas absorption, and
distillation are examples. In other processes, such as drying, crystallization,
and dissolution, one phase may consist of only one component. Concentration
gradients are set up in one phase only, with the concentration at the interface
given by the relevant equilibrium conditions. In drying, for
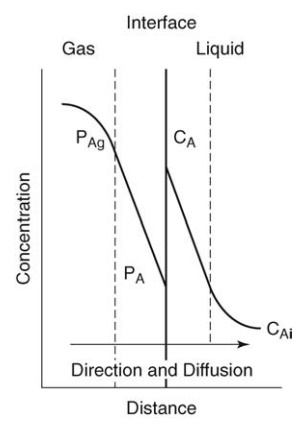
FIGURE 4.3 Interfacial mass transfer.
example,
a layer of air in equilibrium, that is, saturated, with the liquid is
postulated at the liquid surface and mass transfer to a turbulent airstream
will be described by equation (4.5). The interfacial partial pressure will be
the vapor pressure of the liquid at the temperature of the surface. Similarly,
dissolution is described by equation (4.6), the interfacial concentration being
the saturation concentration. The rate of solution is determined by the
difference between this concentration, the concentration in the bulk solution, and
the mass transfer coefficient.
Related Topics
