Immunosuppressant Drugs
| Home | | Pharmacology |Chapter: Essential pharmacology : Immunosuppressant Drugs, Gene Therapy
These are drugs which inhibit cellular/humoral or both immune response and have their major use in organ transplantation and autoimmune diseases.
IMMUNOSUPPRESSANT DRUGS
These are drugs which
inhibit cellular/humoral or both immune response and have their major use in
organ transplantation and autoimmune diseases. The drugs are:
1. Calcineurin Inhibitors (Specific Tcell inhibitors)
Cyclosporine
(Ciclosporin), Tacrolimus
2. Antiproliferative
Drugs (Cytotoxic drugs)
Azathioprine, Cyclophosphamide, Methotrexate, Chlorambucil,
Mycophenolate mofetil (MMF)
3. Glucocorticoids
Prednisolone and
others
4. Antibodies
Muromonab CD3,
Antithymocyte globulin (ATG), Rho (D) immuneglobulin
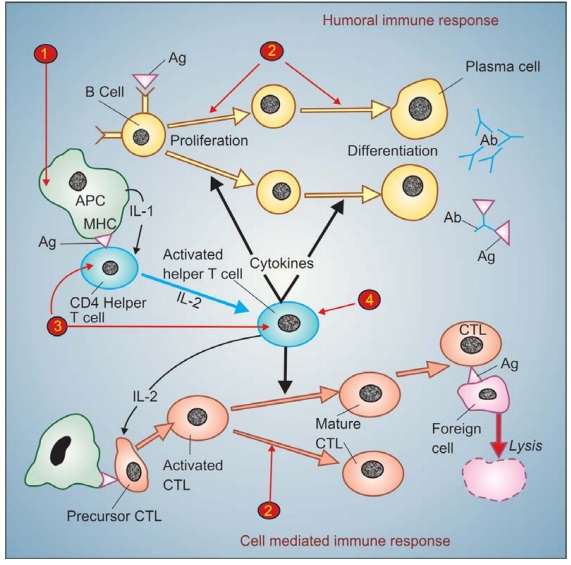
The development of
immune response and the sites of action of different immunosuppressants is
summarized in Fig. 63.1.
CALCINEURIN INHIBITORS
(Specific Tcell inhibitors)
Cyclosporine
It is a cyclic
polypeptide with 11 amino acids, obtained
from a fungus and introduced in 1977 as a highly selective immuno-suppressant
which has markedly increased the success of organ transplantations. It profoundly
and selectively inhibits T lymphocyte proliferation, IL2 and other cytokine
production and response of inducer T cells to IL1, without any effect on
suppressor Tcells. Lymphocytes are arrested in G0 or G1
phase.
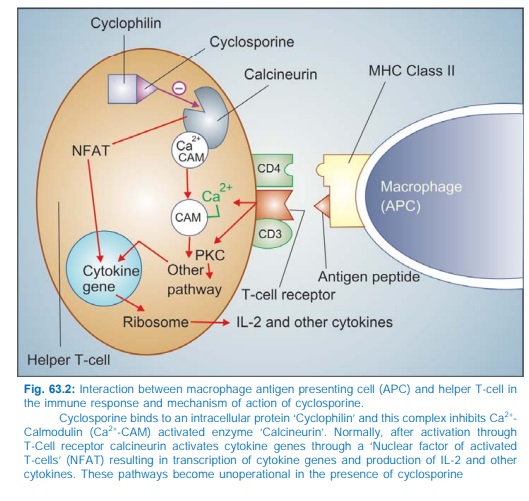
The CD4 molecule
associated with T cell receptor on helper T cells anchors the major
histocompatibility complex class II (MHC II) carrying the antigen peptide so
that it is able to activate the T cell receptor (Fig. 63.2). Stimulation of T
cell receptor produces a cascade of Ca2+ dependent events and protein kinase C
(PKC) activation. The Ca2+ ions after binding to calmodulin activate a membrane
associated serine/ threonine phosphatase called calcineurin which dephosphorylates regulatory protein ‘nuclear
factor of activated Tcell’ (NFAT), permitting its intranuclear migration and transcription
of cytokine genes leading to production of IL2 along with other interleukins,
GMCSF, TNFα, interferon, etc.
Cyclosporine enters target cells and binds to cyclophilin, an immunophilin class of protein. The complex then
binds to and inactivates calcineurin → response of the helper T cell to antigenic
stimulation fails. Cyclosporine also enhances expression of an inhibitor of IL2
which attenuates IL2 stimulated Tcell proliferation and production of killer
lymphocytes. Cyclosporine is most active when administered before antigen
exposure, but can, in addition, suppress the responses of primed helper T
cells; hence useful in autoimmune diseases as well.
Cyclosporine
selectively suppresses cell-mediated immunity, prevents graft rejection and yet
leaves the recipient with enough immune activity to combat bacterial infection.
Unlike cytotoxic immunosuppressants, it is free of toxic effects on bone marrow
and RE system. Humoral immunity remains intact. However, it is nephrotoxic—the
major limitation, and impairs liver function. Other adverse effects are
sustained rise in BP, precipitation of diabetes, anorexia, lethargy, hyperkalaemia,
opportunistic infections, hirsutism, gum hyperplasia, tremor and seizures.
Cyclosporine is the
most effective drug for prevention and treatment of graft rejection reaction.
It is routinely used in renal, hepatic, cardiac, bone marrow and other transplantations.
For induction it is started orally 12 hours before the transplant and continued
for as long as needed. When graft rejection has started, it can be given i.v.,
because oral bioavailability is low, dependent on presence of bile and is
highly variable. It is concentrated in WBCs and RBCs, metabolized in liver by
CYP3A4 and excreted in bile. The plasma t½ is biphasic 4–6 hr and 12–18 hr.
Dose: 10–15 mg/kg/day with
milk or fruit juice till 1–2 weeks
after transplantation, gradually reduced to maintenance dose of 2–6 mg/kg/day.
Therapy may be started with 3–5 mg/kg i.v. infusion.
IMUSPORIN 25, 50, 100
mg soft gelatin cap. Absorption from this preparation
is slower and more variable. A newer microemulsion formulation SANDIMMUN NEORAL, PANIMUN BIORAL 25, 50,
100 mg caps, has more consistent bioavailability. For i.v. use cyclosporine
is dispersed in cremaphor emulsion: SANDIMMUN, PANIMUN 100 mg/ml inj
in 1 ml, 5 ml, 50 ml vial, which is diluted and infused over 4–6 hours. An
acute reaction consisting of chills, fever, bodyache and dyspnoea often occurs
because of the solvent; i.v. cyclosporine is used only in emergency, and is
substituted by oral medication as soon as possible.
Cyclosporine is a second line drug in autoimmune diseases, like
severe rheumatoid arthritis, uveitis, bronchial asthma, inflammatory bowel disease,
dermatomyositis, etc. and in psoriasis, especially to suppress acute
exacerbations. It is often used along with corticosteroids or Mtx. Good results
have been obtained in some cases of aplastic anaemia. For these conditions,
lower doses (2–5 mg/kg/day) are needed and adverse effects are mild. However,
it is not curative and relapses occur when the drug is withdrawn.
Drug interactions with a large number of
drugs occur. All nephrotoxic
drugs like aminoglycosides, vancomycin, amphotericin B and NSAIDs enhance its
toxicity. By depressing renal function, it can reduce excretion of many drugs.
Phenytoin, phenobarbitone, rifampin and other enzyme inducers lower its blood
levels so that transplant rejection may result. On the other hand, CYP3A4
inhibitors erythromycin, ketoconazole and related drugs inhibit its metabolism
to increase bioavailability and cause toxicity. Pot. supplements and K+ sparing
diuretics can produce marked hyperkalaemia in patients on cyclosporine.
Tacrolimus (FK506)
It is a newer immunosuppressant
chemically different from cyclosporine, but having the same mechanism of
action, and is ~100 times more potent. It binds to a different cytoplasmic
immunophilin protein labelled ‘FKBP’, but the subsequent steps are the same,
i.e. inhibition of helper T cells via
calcineurin.
Tacrolimus is
administered orally as well as by i.v. infusion. Oral absorption is variable
and decreased by food. It is metabolized by CYP3A4 and excreted in bile with a
longer t½ of 12 hour. Therapeutic application, clinical efficacy as well as
toxicity profile are similar to cyclosporine. It is particularly valuable in
liver transplantation because its absorption is not dependent on bile. Because
of more potent action, it is also suitable for suppressing acute rejection that
has set in. Hypertension, hirsutism and gum hyperplasia are less marked than
cyclosporine, but tacrolimus is more likely to precipitate diabetes, cause
neurotoxicity, alopecia and diarrhoea. Dose limiting toxicity is renal.
Dose: 0.050.1 mg/kg BD oral
(for renal transplant), 0.1–0.2 mg/kg
BD (for liver transplant).
TACROMUS, PANGRAF 1, 5
mg cap.
ANTIPROLIFERATIVE DRUGS
(Cytotoxic Immunosuppressants)
Certain cytotoxic
drugs used in cancer chemotherapy exhibit prominent immunosuppressant action,
mainly by preventing clonal expansion of T and B lymphocytes (see Fig. 63.1).
Azathioprine
It is a purine antimetabolite
which has more marked immunosuppressant than anti-tumour action. The basis for
this difference is not clear, but may be due to its selective uptake into
immune cells and intracellular conversion to the active metabolite 6mercaptopurine,
which then undergoes further transformations to inhibit de novo purine synthesis and damage to DNA. It selectively affects
differentiation and function of T cells and inhibits cytolylic lymphocytes;
cell-mediated immunity is primarily depressed.
The most important
application of azathioprine is prevention of renal and other graft rejection,
but it is less effective than cyclosporine; generally combined with it or used
in patients developing cyclosporine toxicity. It has also been used in
progressive rheumatoid arthritis and some other autoimmune diseases.
Cyclophosphamide
This cytotoxic drug has more marked
effect on B cells and humoral immunity compared to that on T cells and cell-mediated
immunity. It has been particularly utilized in bone marrow transplantation in
which a short course with high dose is generally given. In other organ transplants
it is employed only as a reserve drug. In rheumatoid arthritis, it is rarely
used, only when systemic manifestations are marked. Low doses are occasionally
employed for maintenance therapy in pemphigus, systemic lupus erythematosus and
idiopathic thrombocytopenic purpura.
Methotrexate (Mtx.)
This folate antagonist is a potent
immunosuppressant which markedly depresses cytokine production and cellular
immunity, and has anti-inflammatory property. It has been used as a first line
drug in many autoimmune diseases like rapidly progressing rheumatoid arthritis,
severe psoriasis, pemphigus, myasthenia gravis, uveitis, chronic active
hepatitis. Low dose Mtx maintenance therapy is relatively well tolerated.
Chlorambucil
It has relatively weak
immunosuppressant action which is sometimes utilized in autoimmune diseases and
transplant maintenance regimens.
Mycophenolate mofetil (MMF)
It is a new immunosuppressant; prodrug of mycophenolic
acid which selectively inhibits inosine
monophosphate dehydrogenase an enzyme essential for de novo synthesis of guanosine nucleotides in the T and B cells
(these cells, unlike others, do not have the purine salvage pathway).
Lymphocyte proliferation, antibody production and cellmediated immunity are
inhibited. As ‘add on’ drug to cyclosporine + glucocorticoid in renal transplantation,
it has been found as good or even superior to azathioprine, but should not be
combined with azathioprine. It can help to reduce the dose of cyclosporine and
thus its toxicity. Vomiting, diarrhoea, leucopenia and predisposition to CMV
infection, g.i. bleeds are the prominent adverse effects.
Dose: 1.0 g BD oral; CELLMUNE, MYCEPT,
MYCOFIT 250, 500 mg tab/cap.
GLUCOCORTICOIDS
Glucocorticoids have potent immunosuppressant and anti-inflammatory
action, inhibit several components of the immune response. They particularly
inhibit MHC expression (Fig. 63.1) and proliferation of T lymphocytes.
Expression of several IL and other cytokine genes is regulated by
corticosteroids and production of adhesion molecules is depressed. The short-lived
rapid lymphopenic effect of steroids is due to sequestration of lymphocytes in
tissues. Accordingly, they have a more marked effect on CMI.
The corticosteroids are widely employed as companion drug to cyclosporine
in various organ transplants. In case graft rejection sets in—large doses of
corticoids i.v. are employed for short periods. They are used in practically
all cases of severe autoimmune diseases, especially during exacerbation. Long-term
complications are the greatest limitations of steroid use.
IMMUNOSUPPRESSANT ANTIBODIES
Muromonab CD3
It is a murine monoclonal antibody against the CD3
glycoprotein located near to the T cell receptor on helper T cells (see Fig. 63.2). Binding of muromonab CD3
to the CD3 antigen obstructs the binding of MHC IIantigen complex to the T cell
receptor: antigen recognition is interfered, so that participation of T cells
in the immune response is prevented and T cells rapidly disappear from
circulation leading to an immune blocked state. The response to this monoclonal
antibody is less variable than to the polyclonal anti-thymocyte globulin. It is
also less likely to produce allergic reactions.
Muromonab CD3 has been used as induction therapy together with
corticosteroids and azathioprine with delayed use of cyclosporine in
‘sequential regimen’ for organ transplantation. This serves to postpone
potential nephro and hepatotoxicity of cyclosporine. This sequential regimen
has been found to be more effective than the standard triple therapy in renal
and hepatic, but not in cardiac transplant recipients. It is also valuable for
steroid-resistant rejection reactions and has been used to deplete T cells from
the donor bone marrow before transplantation.
The initial doses of muromonab CD3 are associated with ‘cytokine
release’ syndrome with flu like symptoms: chills, rigor and wheezing.
Occasionally aseptic meningitis, intragraft thrombosis, pulmonary edema,
seizures and a shock like state are produced. High dose corticosteroid pretreatment
reduces the reaction.
Antithymocyte Globulin (ATG)
It is a polyclonal antibody purified from horse or rabbit
immunized with human thymic lymphocytes which binds to T lymphocytes and
depletes them. It is a potent immunosuppressant and has been used primarily to
suppress acute allograft rejection episodes, especially in steroid-resistant
cases or is combined with them. It can also be used in induction regimens, but
responses are less consistent than with muromonab CD3, and it has the potential
to produce serum sickness or anaphylaxis, but is less expensive than muromonab
CD3.
LYMPHOGLOBULIN
(equine) 100 mg/vial inj.; 10 mg/ kg/day i.v.; THYMOGLOBULIN (rabbit)
25 mg/vial inj.; 1.52.5 mg/ kg/day.
ATG 100 mg inj; 200 mg i.v./day.
Anti-D Immuneglobulin
It is human IgG having a high titer of antibodies against Rh
(D) antigen. It binds the Rho antigens and does not allow them to induce
antibody formation in Rh negative individuals. It is used for prevention of
postpartum/postabortion formation of antibodies in RhoD negative, DU negative
women who have delivered or aborted an RhoD positive, DU positive baby/foetus.
Administered within 72 hours of delivery/ abortion, such treatment prevents Rh
haemolytic disease in future offspring. It has also been given at 28th week of
pregnancy.
Dose: 250–350 μg i.m. of freez dried
preparation. RHIGGAL 100, 350 μg vial, RHESUMAN,
RHOGAM 300 μg/vial inj.
Higher doses (1000–2000 μg) are needed for Rh
negative recipients of inadvertantly administered Rh positive blood. It should
never be given to the infant or to RhoD positive, DU positive individuals.
Related Topics
