Hospital procurement and the application of EU legislation
| Home | | Hospital pharmacy |Chapter: Hospital pharmacy : Purchasing medicines
Overlaid on this background and history has been the impact of EU legislation. The UK is a member state of the EU and an aim of the EU is to create a single European market devoid of all trading restrictions and barriers a marketplace in which all businesses have an equal opportunity to compete.
Hospital procurement and the application of EU legislation
Background and requirements
Overlaid on this
background and history has been the impact of EU legislation. The UK is a
member state of the EU and an aim of the EU is to create a single European
market devoid of all trading restrictions and barriers a marketplace in which
all businesses have an equal opportunity to compete. The EU regulates and
monitors all large-scale public sector procurement through EU directives
covering the supply of goods, services and works. In the UK the directives
apply to all NHS contracting authorities and NHS trusts.
As a result of EU
membership, hospital procurement is subject to the directive within the Treaty
of Rome, including Article 12 (prohibition of discrimination on grounds of
nationality), Article 28 (free movement of goods within the EU) and Article 81
(prohibition of agreements that prevent, restrict or distort competition).
The main
requirements are:
· the advertisement of
large public contracts to a standard format in the supplement to the Official
Journal of the European Community (OJEC) so that suitable suppliers from all EU
and government procurement agreement countries have the opportunity to declare
their interest. Prescribed minimum periods for responses
· the use of technical
specifications which are non-discriminatory and which refer to EU or other
recognised international standards wherever possible
·
the use of objective criteria for selecting participants and
awarding contracts.
The directives only
apply where the value of the procurement exceeds a given threshold. This is
quoted in euros but, as a rule of thumb, means any contract with a value of
£100 000 is included. It should be noted that the figure is for the contract’s
lifetime, not an annual figure; thus, a contract for £30 000 per year for 3
years must go through this process.
Types of procedure
The regulations
recognise three contracting procedures open, restricted and negotiated. These
have slightly different requirements and advantages, and are summarised in Text
box 3.1.
Box 3.1 Types of contracting procedure
Open procedure
Available
in all circumstances and involves only a single stage. All offers received must
be considered, provided that candidates have passed any minimum short-listing
criteria. The open procedure can be conducted more quickly than the restricted
procedure but there is no possibility of limiting the number of bids received.
Restricted procedure
Available
in all circumstances but involves a two-stage procedure. From amongst the
candidates expressing interest (the first stage) it is possible to shortlist a
limited number from whom to invite offers (the second stage).
Negotiated procedure
The most flexible but the least transparent of the three procedures. It is used only in very limited circumstances (for example, where goods are needed urgently due to reasons that were unforeseeable by, and not attributable to, the buyer).
Offer evaluation
The directives
require that any contract must be awarded to the candidate who submits the lowest-priced
tender or the tender that is the most economically advantageous (buyers almost
invariably select the latter because it gives greater flexibility). The factors
that may be used to determine economic advantage include price, quality of
service and running costs: the chosen factors must be stated in the OJEC notice
or the contract documents.
Negotiation within the process
Where the open or
restricted procedures are being used, the rules forbid buyers to engage in
post-tender negotiations with candidates. These are defined as negotiations
with candidates on fundamental aspects of their bids, for ex-ample, price.
Discussions aimed as clarifying or supplementing the content of the bids are,
on the other hand, permitted, provided all candidates are treated equally.
Pre-tender discussions with potential suppliers, conducted on an equit-able
basis, are critical to designing contracts that will perform and deliver.
Types of contract
There are two types
of contract: the commitment contract and the framework contract. The commitment
contract commits a legal entity (such as an NHS trust) to purchase a defined
quantity of product at a defined price; the frame-work contract does not
guarantee to deliver commitment. Rather, based on estimated volumes it provides
(for example, on behalf of a group of hospitals represented as a purchasing
group) a framework against which purchase orders will be placed by hospitals
covered by the agreement. The framework agreement sets the terms and conditions
of the purchase by the hospital, including price/pricing schedules, with the
trust contract being formed when individual hospitals place their purchase
order.
Framework contracts
are normally used on behalf of purchasing groups in recognition that an agent
(such as the NHS CMU) or a hospital within the purchasing group cannot deliver
absolute commitment to volume on behalf of the group.
The strengths of the contracting process
It may appear
sometimes that the contracting process is cumbersome and bureaucratic. However,
recognition must be paid to its inherent strengths. These are that the process
is auditable, is legal (so minimising the risk of challenge, particularly when
the lowest bid is not accepted), provides a frame-work for equal treatment for
all bidders, establishes a clear trading basis between the NHS and its
suppliers (through standard terms and conditions) and provides a fair test of
value for money on behalf of the NHS.
The organisation of hospital contracts
Hospitals have
contracts at various levels: purchasing group, trust con-tracts, national. The
principles of purchasing group contracts are straight-forward. Hospitals
aggregate their purchasing power through their pharmacy purchasing groups and
the NHS CMU then competitively tenders, awards and manages the resulting
contracts, as an agent, on behalf of the groups. Each trust must nominate an
individual to represent the interests of its hospital managers, clinicians and
budget-holders (as well as its local relationships with primary care trusts) on
its purchasing group. The nominee’s roles include sharing information,
adjudicating contracts and participating in collective dialogue with the NHS
CMU buyer dedicated to work with the group.
The nominees use
their knowledge and experience that originate in man-aging medicines on a
day-to-day basis (particularly through formulary management systems that are
linked to drugs and therapeutic committees, and input into the prescribing
process) to direct the management of the contracts.
In England there are
six main purchasing groups operating on a geographical basis with some, varying
by main group, being divided into smaller groups. Each main group ‘owns’
contracts of between 1500 and 2000 lines, representing upward of 200 suppliers.
Whilst the contracts on behalf of these groups are framework contracts that do
not guarantee commitment, the ownership of the contracts (through the
participation of their member trusts) ensures that these contracts are highly
effective.
The framework
contracts on behalf of each purchasing group can vary in length. However,
typically they last for a maximum period of 2 years and include options to
extend for an additional 2 years.
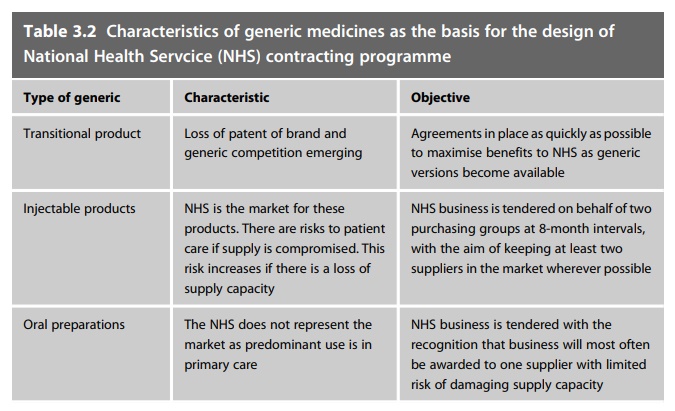
Following SCEP the
NHS now supports a nationally coordinated programme to contract for the supply
of its generic medicines. This is organised to reflect the characteristics of
the generics that are involved and is sum-marised in Table 3.2.
Alongside the
generic contracting programme there are tenders for branded medicines in the
same way but by local agreement with NHS CMU, making awards to reflect volume
discounts. This enables the NHS to benefit from competition between
therapeutically similar medicines where this exists. Some product ranges can be
separated out from the model to reflect the strategic development of their
procurement; these are shown in Table 3.3.

Table 3.3 Specific contracting activities for
certain product type
Product range : Contract
Infection control products : By
National Health Service supply chain to enable direct-to-ward supply
Clotting factors : On
behalf of haemophilia centres through a direct relationship
Immunoglobulins : Through
a single national tender; to match supply against commitment by purchasing
group
Vaccines : Through commitment
contracts on behalf of the Department of Health to support the delivery of its
national vaccination programmes
Pharmacy purchasing groups branded medicines and other activities
Contracts at
purchasing group level are more important when decisions around the clinical
choice of medicines can be influenced at a local level. Product ranges
contracted for at this level include: branded medicines; bulk fluids;
therapeutic tenders (where branded medicines have the same therapeutic outcome);
service contracts (for example home care, aseptic com-pounding and
overlabelling services).
Trust contracts
Some medicines will
still be contracted for at trust level, though the driver is to move as much
contracting to purchasing group and national level as possible, where
aggregation of usage improves purchasing power.
Systems and processes
Pharmacy specialists and working relationships with procurement
The technical and
procurement specialists within pharmacy strengthen the contracting
arrangements, especially where they are employed by a trust to support and work
with a purchasing group. Pharmacy quality control (QC) arrangements are
involved in assessing product quality as part of the tendering process, as well
as on a day-to-day basis (see Chapter 7 for further details on QC services).
Specialist
involvement, combined with day-to-day working relationships between trusts and
their buyer, minimises duplication of effort through shared access to central
contract management and associated procurement expertise.
NHS CMU management systems
NHS CMU manages the
contracting process using a system called Phacter. Phacter maintains a database
of all suppliers and product lines. It generates invitations to tender (through
an electronic format that suppliers access through an electronic portal Bravo)
and produces comparative evaluations to support contract adjudication before
finally generating award notices to suppliers and contract details to trusts
through a web-accessed catalogue.
NHS CMU also
collects, at each month end, in electronic format, hospital pharmacy purchasing
information through a system known as Pharmex. Pharmex data are used to scope
NHS secondary care business for tender. They also provide individual trusts and
NHS CMU with measures reporting the performance of the contracting
arrangements.
Through a third
system, PharmaQC, NHS CMU collects and stores product images and supply chain
information from suppliers. The QC pharmacists access this system to record
their product assessments. Available at adjudication, via the system, this
information ensures that QC product quality and risk assessments are reflected
when contracts are awarded.
Meeting structures
The totality of the
contracting arrangements is underpinned through meeting arrangements and
communication structures. The pharmacy purchasing groups, elected chairs and
their buyers meet regularly to share information, adjudicate contracts and
monitor performance. At the national level the Pharmaceutical Market Support
Group (PMSG), consisting primarily of the specialist procurement pharmacists
and NHS CMU specialists, brings pharmaceutical expertise around a focus of
contract management, making sure that security of supply is placed above
savings. PMSG is supported by various working groups dedicated to specific
workstreams. It reports to the National Pharmaceutical Supply Group (NPSG).
NPSG membership consists of NHS trust chief pharmacists and PCT advisors. Its
role is to provide advice to NHS CMU to ensure that it manages and develops its
service in line with NHS requirements. Lastly, the chairs of both NPSG and PMSG
attend regular meetings with the Department of Health chief pharmacist, the NHS
CMU general manager, amongst others, to ensure that there is an exchange of
information with Department of Health col-leagues working at policy level.
Recent changes
All of the following
changes will have long-term impact.
Home care
The growth in the
supply of medicines to patients at home, either as compo-nents of packages of
care or just simply as a route of supply, has been dramatic. Home care now
represents a major part of NHS business. The NHS focus lies with the National
Homecare Committee.
Other service contracts
Stimulated by
policy, by National Patient Safety Agency guidance and increasing demands on
its finite capacity, the NHS is increasingly outsourcing other services such as
outpatient dispensing, the provision of aseptically prepared products and
‘specials’ in addition to home care. Over time these changes will have an
impact on contracting and procurement.
Payment by results and NICE
As described in
Chapter 1, for England, payment by results has started to change the dynamics
within the market for those high-cost medicines that are not included in the
payment by results tariff (and particularly those approved by the National
Institute for Health and Clinical Excellence (NICE): see Chapter 11). The PPRS
2009 allows pharmaceutical companies to pro-pose patient schemes to improve the
cost-effectiveness (cost per quality-adjusted life-year) of medicines. If NICE
approves or partially recommends the medicine, these schemes become operational
in order to achieve the cost-effectiveness approved by NICE. Types of scheme
include free stock, rebates, straight discounts (applied to invoices at order
point) and dose caps. Whilst providing access via the NHS to a wider range of
medicines at cost-effective rates, these schemes have added a not
inconsiderable burden to NHS medicines purchasing teams.
Security of supply
Globalisation within
the pharmaceutical industry, associated with company mergers, rationalisation
of manufacturing capacity, product discontinua-tions, extended supply chains,
strengthened regulation and shifts in the sour-cing of active pharmaceutical
ingredients from China, are all contributing to increasing stresses within the
supply chain, increasing risks to supply. The risks associated with the supply
of counterfeit medicines are also increasing. The Department of Health
(supported by the Medicines and Healthcare products Regulatory Agency), the
PMSG, NHS CMU and the trade associations all work together to minimise these
risks.
Product coding
The establishment of
the Dictionary of Medicines and Devices, its acceptance and increasing
application mean that the NHS has, for the first time ever, access to
nationally recognised coding and product descriptions. Linked to bar coding
(GS1), which is currently underutilised within hospital pharmacy, this will
create unprecedented opportunities to improve hospital pharmacy supply management.
Local arrangements
The majority of
pharmacy departments now use information technology systems for ordering, goods
receipt and invoice processing. These systems are configured so that the audit
requirement for segregation of these tasks between different staff members is
delivered. Procedures must be consistent with the trust’s standing financial
instructions. Manual systems are occasionally used but these will be phased out
and will not be covered here.
Ordering
Items which need to
be ordered will be identified by the computer system or by pharmacy staff.
Computer systems maintain live stock levels and, as these fall to the reorder
level, the item is flagged for reorder. The reorder level is either fixed or
can be calculated by the system based on an algorithm of average daily usage,
time it takes to be delivered (lead time) and a preset safety factor.
Infrequently used items may be flagged so that they are only placed on order by
authorised staff.
The system will
allocate these items to a preferred supplier that will be one of the following:
· the contract holder
· the manufacturer
offering an NHS price
· a short-line store
· a wholesaler
These lists of items
will be reviewed and amended by an authorised mem-ber of staff and orders
generated. The supplier may be charged if the lead time is not appropriate for
patient needs or the preferred supplier is out of stock. The orders will be
sent to the supplier by one of the following methods:
· verbally by phone
this method is useful if the item is urgent or patient-specific. If used
routinely, verbal ordering is labour-intensive and subject to transcription
errors
· faxing: this reduces
transcription errors but requires re-entering of data by the supplier and is
dependent on the quality of the faxed copy
· electronic data
interchange: this is exchange of electronic order data. It is the objective of
all NHS ordering. The accuracy is dependent on the upkeep of product codes and
so on, but it has the potential for rapid, accurate transfer, with minimal time
commitment for staff. Examples include use of the Pharmacy Messenger system and
Medecator
· post: this route is
now rarely used due to time delay and cost.
Goods receipt
On receipt, goods
will be checked visually for damage and expiry date. They will then be checked
against the delivery note and against either a hard copy or computer copy of an
order. The aim is to ensure that quantities and products are correct and that
there are no obvious defects. Any discrepancies will be notified to the
supplier immediately. Many trusts collect data on errors and timeliness of
deliveries since supplier performance is a key consideration at contract
adjudication. Once checks are complete, items will be entered into the computer
system and stock levels updated.
Batch number details
are recorded in some trusts, although the benefit of this is reduced by the
inability to track batches to the end-user. There is a requirement to do this
with blood products and medicines used in reproductive health services.
When controlled drugs
are ordered and receipted, the requirements of the Misuse of Drugs Regulations
must be followed. Records of receipt are made in a register and the balance of
stock received updated (see Chapter 5 for a more detailed discussion of
controlled drugs)
Invoicing
Practice varies
between trusts: this function is carried out by pharmacy staff in approximately
80% of trusts and by finance staff in the remainder. Wherever it is carried
out, the system requires input of invoice data into the computer system and checking
of price invoiced against price expected on the original order. Most trusts
require exact matching of contract line prices and agreed tolerances with
non-contract prices but use last purchase price on the orders.
Historically,
invoices have been received in hard copy with details manually entered into the
computer system. The receipt of electronic invoice files and subsequent
matching of items is developing rapidly and is used to some extent in 20% of
trusts. These systems automatically process items with complete matching of
data and allow trust staff to focus on price or delivery discrepancies.
Acceptance of
invoice details will update the unit cost of the item(s) on the computer system
and also authorise payment to the supplier.
