Heterolytic Bond Formation
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Curved-Arrow Notation
The formation of a bond between two atoms can proceed by one of the atoms donating an electron pair and the other atom accepting the electron pair.
HETEROLYTIC BOND FORMATION
The
formation of a bond between two atoms can proceed by one of the atoms donating
an electron pair and the other atom accepting the electron pair. As before,
charge must be conserved and the loss and gain in electrons by the donor and
acceptor, respectively, must be accompanied by a corresponding change in formal
charge.
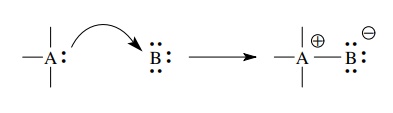
The
atom donating the electron pair must obviously have an electron pair that is
not tightly bound and thus is available for donation. Commonly, lone pairs and π-bonded
electron pairs can be most easily donated, but occasionally electron pairs in σ bonds can be donated if the σ bond is weak or electron rich.
The
atom accepting the electron pair must have an unfilled orbital available which
the donated electron pair can populate. This can be an unfilled valence shell
orbital, as is the case if the acceptor atom has a valence sextet, or it can be
an accessible antibonding orbital, either σ
∗ or π∗.
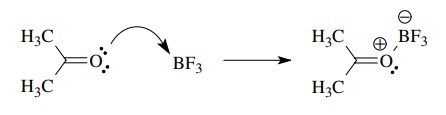
Thus
the reaction of acetone with BF3 is a Lewis acid – base reaction in
which a lone pair of the ketone oxygen atom is donated to an unfilled valence
orbital of BF3. Bond formation is accompanied by the development of
formal charges on both oxygen and boron.
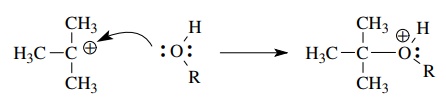
The
capture of carbocations by alcohols involves a similar donation of a lone pair
of electrons on oxygen to the vacant 2p atomic orbital of the sp2-hybridized,
sextet carbocation. Note that charge must be conserved so the first formed
product is a positively charged oxonium ion.
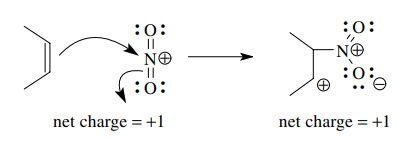
Reaction
of an alkene with a nitronium ion involves donation of the π-electron pair of the alkene into a π∗ orbital of the
nitronium ion. Donation of a bonded electron pair necessarily means that the
bond from which it comes is broken. Likewise population of an antibonding level
by electron donation generally results in breaking of the bond to which the
antibonding orbital corresponds. In this case electron donation of the olefinic
π-electron pair results in the
rupture of the olefinic π bond and
acceptance into the N–O π∗ orbital results in breakage of the N–O π bond as well. Note that a new bond is formed and net charge is
conserved.
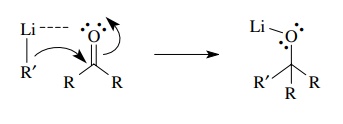
Addition
of an alkyl lithium reagent, which has a very electron rich σ bond between carbon and lithium, with
a ketone involves electron donation of the σ
electrons to the π∗ orbital of the ketone to give a new carbon – carbon bond.
The lithium counterion also plays a role in the addition by complexing with the
lone pairs of the carbonyl oxygen, thus making the carbonyl group more electron
deficient. Effectively this lowers the energy of the π∗ orbital so its
energy better matches the energy of the electron donor, and donation of
electrons into that orbital is facilitated.
It
is important to remember the requirements for any two-electron bond-making
process: first, there must be an available pair of electrons to be donated and,
second, there must be an unoccupied orbital of suitable energy available into
which the electrons can be donated.
Related Topics
