Heterolytic Bond Cleavages
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Curved-Arrow Notation
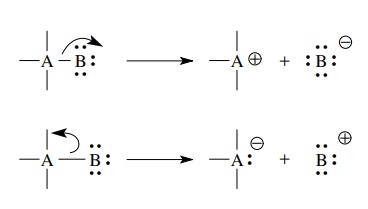
Simple bond cleavages can proceed with the shared pair ultimately residing on either of the previously joined elements. In either case one atom has a sextet electronic structure and a positive charge.
HETEROLYTIC BOND CLEAVAGES
Simple
bond cleavages can proceed with the shared pair ultimately residing on either
of the previously joined elements. In either case one atom has a sextet
electronic structure and a positive charge. The other has a valence octet with at
least one lone pair (the pair that was formerly shared) and a negative charge.
Such bond cleavage, in which the previously shared pair goes with one of the
bonded atoms, is termed heterolytic
cleavage and necessarily results in the formation of charged species.
In
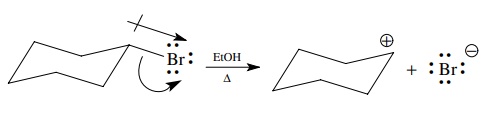
general, the movement of electrons during heterolytic cleavage follows the
direction of bond polarity. In a polar covalent bond the shared pair is
displaced toward the more electronegative element. Upon cleavage the pair of
bonded electrons are transferred completely to the more electronegative
element, which becomes negatively charged, and the more electropositive element
loses the bonded electron pair and becomes positively charged.
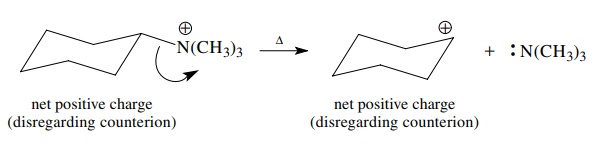
If
one of the bonded elements is positively charged to begin with, it can gain the
bonded pair upon bond cleavage and become neutral. Note, however, that net
charge is always conserved in any reaction. Moreover, bond cleavages are
depicted the same whether they involve σ
or π bonds.
Related Topics
