Hard Capsules
| Home | | Pharmaceutical Technology |Chapter: Pharmaceutical Engineering: Solid Dosage Forms
Hard capsules have traditionally been manufactured from gelatin. The gelatin is obtained from bone or skin (calf or pig) acid or alkali treatment over a period of weeks, in some cases as long as 30 weeks (pork skin, 1–5% HCl).
HARD CAPSULES
Hard capsules have
traditionally been manufactured from gelatin. The gelatin is obtained from bone
or skin (calf or pig) acid or alkali treatment over a period of weeks, in some
cases as long as 30 weeks (pork skin, 1–5% HCl). The product pH is adjusted,
and a hot water extraction is followed by filtration, concentration, and
solidification. The final product is milled to size.
Capsule
shells are prepared by dipping manganese bronze pins into a bath of molten
gelatin. Once removed from the bath, the gelatin solidifies on the pins. The
caps and bodies are then dried and trimmed. Colorant or titanium dioxide (for
opacity) is added as part of this process.
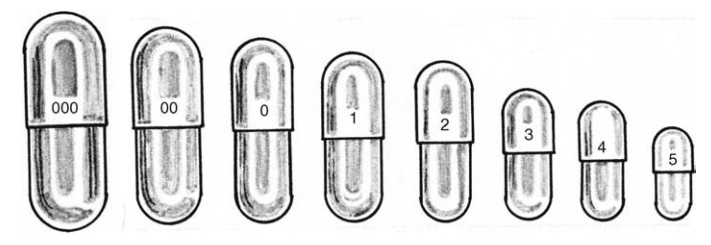
FIGURE 14.3 Different capsule sizes.
The
need for capsules with different physicochemical properties, to aid in
stability, for example, has promoted a search for alternative materials. In
addition, individuals who, for strict religious or health reasons, cannot
ingest gelatin, need alternative products. In this regard, starch and hydrox-ypropylmethyl
cellulose (HPMC) have been developed. There is no reason to believe that other
film-forming polymers might not be useful in this regard. One significant issue
that must be considered is the moisture content of the capsule. Gelatin is known
to optimally contain 5% to 15% moisture. Below 5%, the shell becomes brittle
and may shatter. Above 15% the gelatin distorts, and the shape of dosage form,
if not its integrity, is challenged. The presence of a nutrient-rich
environment and moisture may offer an ideal situation for microbial growth and
enzyme action. Control of microbial growth is, therefore, a serious
consideration in the preparation of capsule products.
Various
capsule sizes are manufactured, as shown in Figure 14.3. There are no strict rules
for predicting required capsule size. Capsules are selected on the basis of
their capacity and the nature of the formulation to be added. The bulk density
and compressibility of the product (drug and excipients) dictate the quantity
of drug that can be placed within a capsule of known volume. Since the drug
dose required to achieve a therapeutic effect can be estimated for new
compounds and is known for existing compounds, this information can be used in
conjunction with the capsule volume to select an appropriate size.
The
requirements for capsule production depend on the scale of manu-facturing.
Extemporaneous preparation (6–12 capsules) usually employs enough of the
product formula to fill one more capsule than required, to account for loss of
fill in manipulation. Special consideration should be given to controlled
substances where all of the drug must be accounted for. Industrially
(thou-sands), the amount necessary to fill the desired number can be prepared
because the error will be small on such a large scale. The operations involved
in large- or small-scale capsule filling are the same. The capsules as supplied
in random orientation must be rectified into a bodies-down, caps-up
orientation. The two shells are then separated, and the capsule is filled with
product formulation. Various methods are available to fill the capsules. For
small-scale production, a plate or single capsule filling method is employed.
On a larger scale, tamping, intermittent compression, continuous compression
vacuum, or auger filling may be employed (Fig. 14.4).
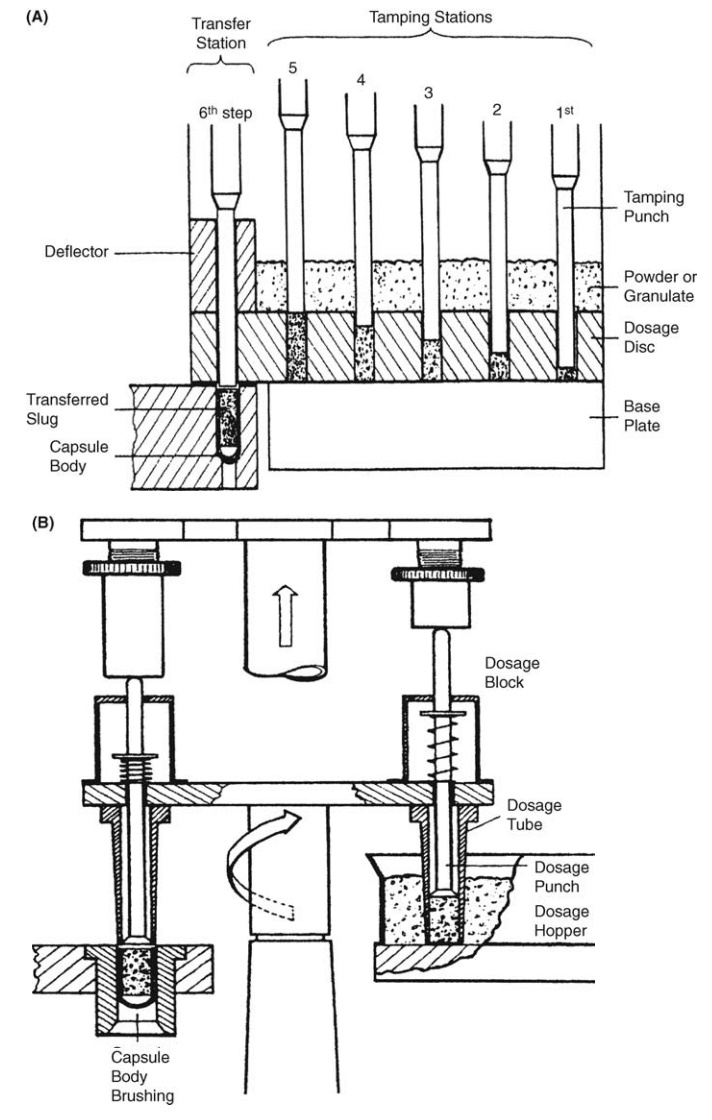
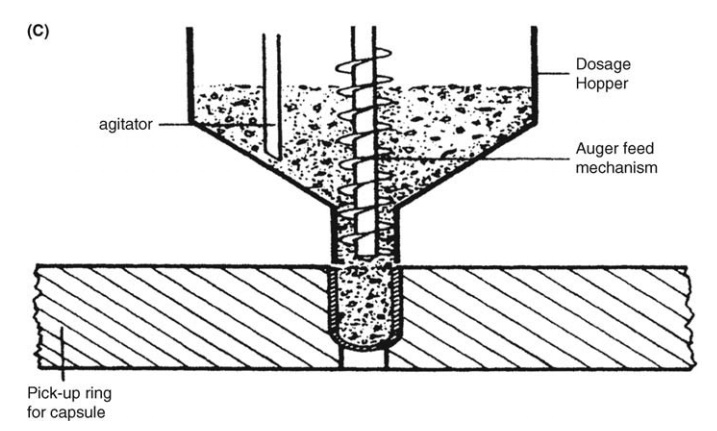
FIGURE 14.4 Capsule filling: (A) tamping,
(B) compression, and (C) auger filling.
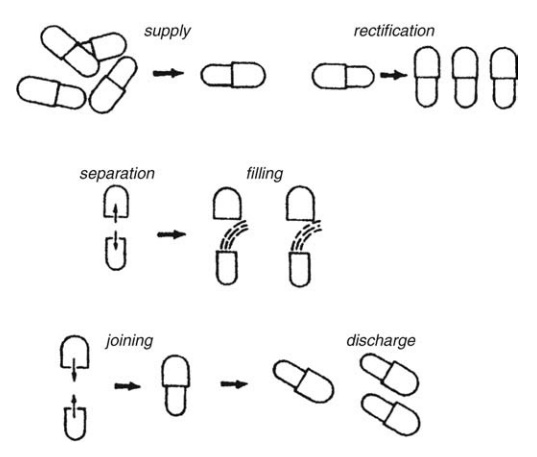
FIGURE 14.5 Capsule manufacturing process.
The shells are then joined and sealed, and the com-pleted product is discharged, as shown in Figure 14.5. Different locking mechanisms have been developed for capsules shown in Figure 14.6. A cleaning and polishing step also follows the manufacturing procedure to improve product appearance.
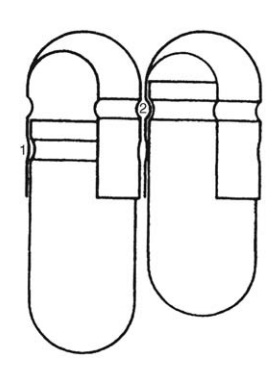
FIGURE 14.6 Capsule locks.
The product is visually inspected following production, its potency and uniformity are evaluated, and it is transferred hygienically to the final packaging.
If the product is hygroscopic, it may be necessary to package cap-sules with
desiccant to avoid moisture uptake. Alternatively, impervious packaging
materials, such as aluminum blisters, may be used. Capsules are easier to
prepare than tablets, are quite flexible with respect to dose, and are easily
combined with other solid dosage forms since other capsules or tablets can be
incorporated into larger capsules.
