Generation of Nucleophilic Carbon Reagents
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Carbon-Carbon Bond Formation Between Carbon Nucleophiles and Carbon Electrophiles
The three major classes of nucleophilic carbon species are organometallic com-pounds, enolate derivatives and related carbanionic compounds, and neutral enol derivatives:
GENERATION OF NUCLEOPHILIC
CARBON REAGENTS
The
three major classes of nucleophilic carbon species are organometallic
com-pounds, enolate derivatives and related carbanionic compounds, and neutral
enol derivatives:
1.
Organometallic compounds which contain a carbon–metal bond are the most
reactive carbon nucleophiles. In most cases they are also powerful bases and
must be prepared and used under strictly anhydrous and aprotic conditions. A
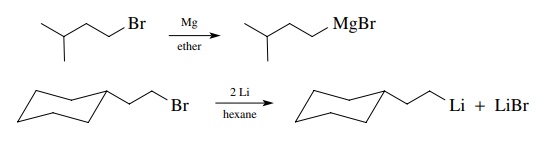
very common way to produce organometallic compounds is to reduce alkyl halides
with active metals. Grignard reagents and organolithium compounds are routinely
produced in this manner. The transformation is a two-electron reduction of the
alkyl halide to a carbanion equivalent; the metal is oxidized.
This
procedure works well for alkyl, vinyl, and aryl halides and provides a
con-venient source of organomagnesium halides and organolithium compounds. In
addition, a variety of other metals can be exchanged for lithium in
organolithium compounds to give different organometallic compounds of modified
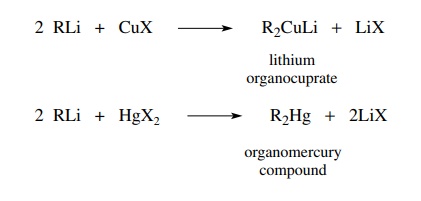
reactivity. Reaction of two equivalents of an organolithium compound with a
cuprous halide gives a lithium organocuprate in which the carbon–lithium bonds
of the organolithium reactant are converted to carbon–copper bonds in the
anionic organocuprate. Lithium merely serves to balance the charge of the
organocuprate. By a similar exchange, dialkylmercury compounds can be prepared
from organo-lithiums and Hg[II] halides.
A
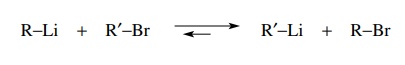
second way to make organometallic compounds for use as carbanion nucleophiles
is to use halogen–metal exchange. In this process an alkyl halide and an
organometallic compound undergo a metathesis reaction to give a new
organometallic compound and a new alkyl halide. This process is thought to take
place by nucleophilic attack on the halogen atom by the organometallic reagent.
One
requirement is that the pKa
of the new organometallic compound is lower than the pKa of the starting organometallic. This in essence means
that the equilibrium is driven to products by the formation of a more stable
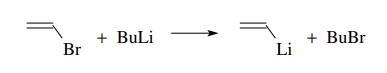
anion. This method is commonly used to make vinyl lithiums from vinyl halides
and alkyl lithiums and aryl lithiums from aryl halides and alkyl lithiums
because the electron pair in an sp2 orbital of a vinyl or aryl
lithium compound is more stable than the electron pair in an sp3
orbital of an alkyl lithium.
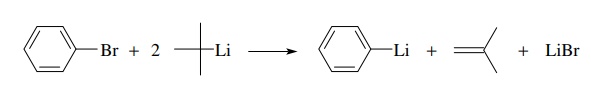
A
method often employed to drive the halogen–metal exchange equilibrium to
completion is to employ tert-butyl
lithium as the organolithium component. In addition to being the most basic
organolithium compound because of the tertiary substitution, conversion of the tert-butyl bromide by-product to
isobutylene also occurs under the reaction conditions and drives the exchange
equilibrium to completion. Note that two equivalents of tert-butyl lithium are required as one equivalent is used in the
halogen–metal exchange and one equivalent is consumed in converting tert-butyl bromide to isobutylene.
2.
Enolates and related carbanionic nucleophiles are routinely generated by
removal of an acidic proton in a molecule with a base. Carbonyl groups acidify
their α protons somewhat and make
their removal by a base a common process. However, structural features other
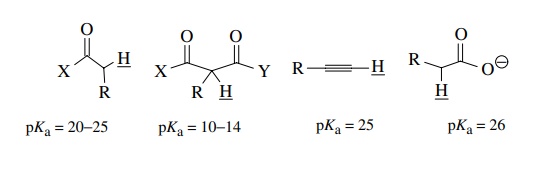
than carbonyl groups can also acidify protons bound to carbon and thus
facilitate their removal by bases. For example, pKa values for structurally acidified C–H protons include
the ones given below.
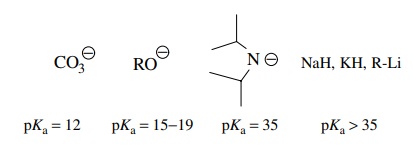
The
pKa’s of commonly used
bases are as follows:
By
knowing (or estimating) the pKa
of a proton to be removed, it is possi-ble to choose a base with a higher pKa in order to have
essentially complete conversion to the anionic carbon nucleophile. When these
conditions are met, proton exchange occurs readily and a carbon nucleophile is
produced. It must be remembered, however, that many bases can serve as nucleophiles.
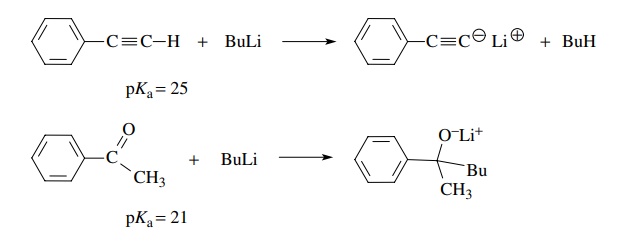
If the structural feature which acidified the C–H proton is an electrophile,
then a nucleophilic base cannot be used. For example, butyl lithium (pKa > 45) converts phenylacetylene (pKa ∼ 25) smoothly to its
conjugate base by proton removal, whereas it reacts as a nucleophile with the
carbonyl group of acetophenone in spite of the fact that the α protons of acetophenone have pKa = 21 and are thus more acidic than
the terminal proton in phenylacetylene.
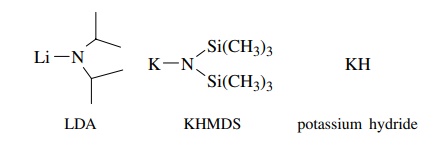
To
circumvent problems of nucleophilicity, lithium diisopropylamide (LDA),
potassium hexamethyldisilylamide (KHMDS), and KH are often employed for proton
removal since they are very strong bases (pKa
> 35) but relatively poor
nucleophiles. Hence they remove protons from acidic C–H bonds but normally do
not attack carbonyl groups or other electrophilic centers.
If
the C–H proton is highly acidified as in a β-dicarbonyl
compound (pKa ∼
10–14)
or nitro compounds (pKa = 9–12), weaker bases such as
alkoxides (pKa ∼
17) can be used to convert the material completely to its conjugate base, and
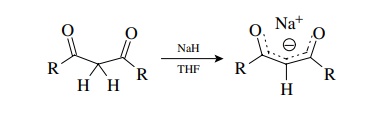
thus aprotic conditions are no longer required. However, a common protocol to
convert dicarbonyl compounds to their enolates in a clean, controllable manner
is to use sodium hydride in dry THF.
3.
A third major class of carbon nucleophiles is enol derivatives. In general,
these are stable compounds that are prepared by one of the functional group
transformations outlined in the previous chapter.
Related Topics
