General Principles in Chemotherapy of Cancer
| Home | | Pharmacology |Chapter: Essential pharmacology : Anticancer Drugs
Bacterial metabolism differs markedly from that of the host, while malignant cells are in fact host cells with deranged regulation of growth and differentiation and only minor other differences.
GENERAL PRINCIPLES IN CHEMOTHERAPY
OF CANCER
1. In cancer chemotherapy, analogy is drawn with bacterial
chemotherapy; the malignant cell being viewed as an invader. However, there are
two main differences—
a) Bacterial metabolism differs markedly from that of the host,
while malignant cells are in fact host cells with deranged regulation of growth
and differentiation and only minor other differences. Therefore, selectivity of
drugs is limited. A number of measures which enhance selectivity of drugs for
the tumour need to be exploited. However, lately some unique tumour antigens
and oncogenes (like the CML-tyrosine protein kinase gene) have been identified,
which provide specific targets for drug therapy.
b) Infecting microorganisms are amenable to immunological and
other host defence mechanisms. This is absent or minimal against cancer cells.
Human interferon α-2 and other cytokines
(interleukin2, tumour necrosis factor, etc.) that can modify the biological
responses to tumour cells are being used as adjuvants in treating neoplasms.
They appear to have some direct inhibitory effect on malignant cells, in addition
to reinforcing immunological defence against these.
2. A single clonogenic
malignant cell is capable of producing progeny that can kill the host. To
effect cure, all malignant cells must be killed or removed. Survival time is
related to the number of cells that escape chemotherapeutic attack.
3. In any cancer,
sub-populations of cells differ in their rate of proliferation and
susceptibility to cytotoxic drugs. These drugs kill cancer cells by first order
kinetics, i.e. a certain fraction of cells present are killed by one treatment.
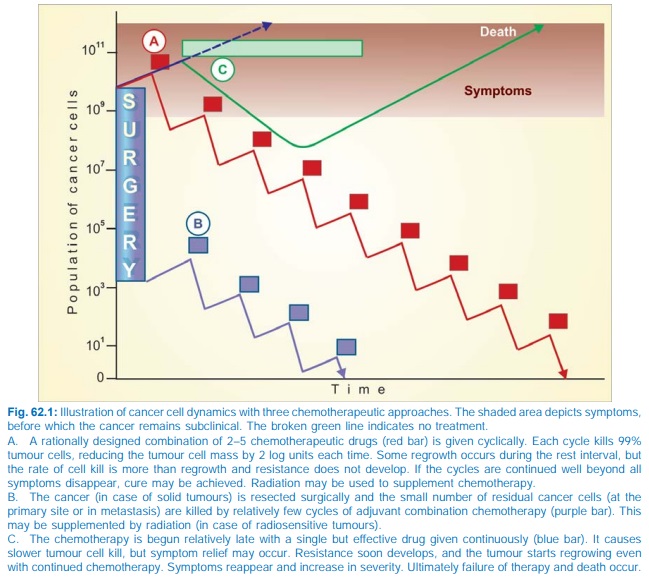
4. Drug regimens or
number of cycles of combined chemotherapy which can effectively palliate large
tumour burdens may be curative when applied to minute residual tumour cell
population after surgery and/or irradiation. This is the basis of the combined
modality approach (see Fig. 62.1).
5. Whenever possible,
complete remission should be the goal of cancer chemotherapy: drugs are often
used in maximum tolerated doses. Intensive regimens used earlier yield better
results.
6. Formerly cancers
were treated with one drug at a time. Now a combination of 2–5 drugs is given
in intermittent pulses to achieve total
tumour cell kill, giving time in
between for normal cells to recover
(Fig. 62.1).
Synergistic
combinations and rational sequences are devised by utilizing:
·
Drugs which are effective when used alone.
·
Drugs with different mechanisms of action.
·
Drugs with differing toxicities.
· Empirically by trial and error; optimal
schedules are mostly developed by this procedure.
·
Drugs with different mechanisms of resistance.
·
Drugs with known synergistic biochemical
interactions.
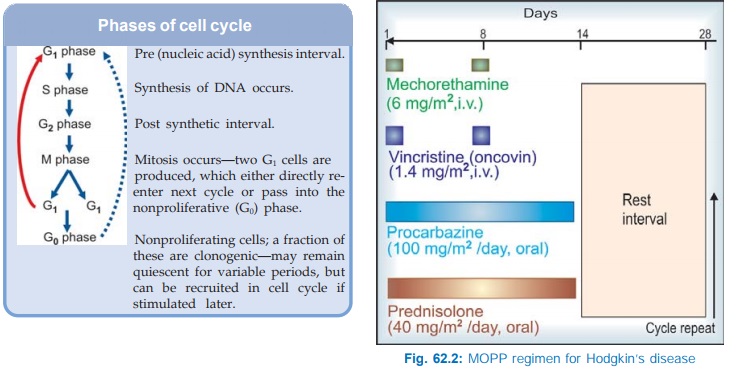
· Kinetic scheduling: On the basis of cell
cycle specificity/nonspecificity of
the drugs and the phase of cell cycle (see
box) at which the drug exerts its toxicity.
Cytotoxic drugs are either cell cycle specific (CCS) or cell
cycle nonspecific (CCNS).
Cell Cycle Nonspecific: Kill resting as well as dividing cells, e.g.
nitrogen mustard, cyclophosphamide, chlorambucil, carmustine, dacarbazine,
busulfan, L-asparaginase, cisplatin, procarbazine, actinomycin D.
Cell Cycle
Specific: Kill only actively dividing cells. Their toxicity
is generally expressed in S phase. However, these drugs may show considerable
phase selectivity, e.g.—
G1:
Vinblastine.
S : Mtx, cytarabine, 6TG, 6MP, 5FU, hydroxyurea, mitomycin C,
doxorubicin, daunorubicin.
G2: Daunorubicin, bleomycin, etoposide, topotecan.
M: Vincristine, vinblastine, paclitaxel,
docetaxel.
Phases of
cell cycle
It is logical to use
cell cycle specific drugs in short courses (pulses) of treatment. This allows
noncycling cells (which are generally less susceptible to drugs) to reenter the
cycle between drug courses. The CCS drugs are generally scheduled after a
course of CCNS drug(s) to improve the cell kill. The CCS drugs are more
effective in haematological malignancies and in solid tumours with a large
growth fraction, while the CCNS drugs are effective in these as well as in
solid cancers with a small growth fraction.
Many regimens have
been devised by taking into consideration the above factors and by observing
patient response.
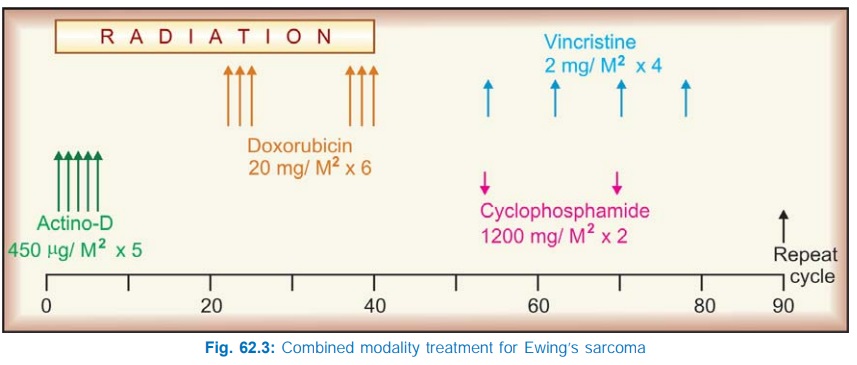
One popular combination has been the MOPP regimen, which has yielded over 80% response rate in Hodgkin’s disease. It is illustrated in Fig. 62.2. For optimum remission 6–11 cycles may be needed. Maintenance therapy thereafter does not produce additional benefit.
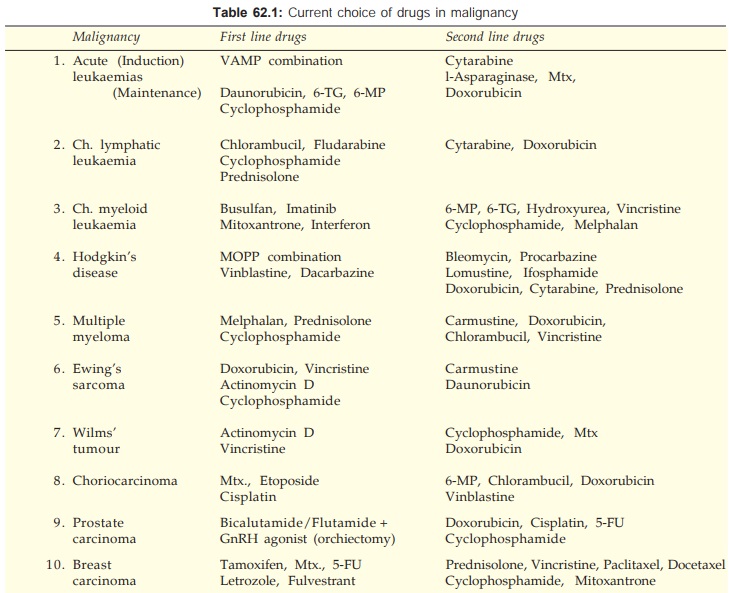
Another combination
that has produced almost 100% response in Ewing’s sarcoma is illustrated in
Fig. 62.3.
Similarly many other
regimens have been devised for different tumours.
VAMP: Vincristine + Amethopterine (Mtx) + 6MP + Prednisolone:
used in acute leukaemia.
COAP: Cyclophosphamide + Oncovin (Vincristine) + AraC (Cytarabine)
+ Prednisolone.
POMP: Prednisolone + Oncovin + Mtx + Purinethol (6MP).
CART: Cytarabine + Asparaginase + Rubidomycin (Daunorubicin) +
6TG.
BACOP: Bleomycin
+ Adriamycin (Doxorubicin) + Cyclophosphamide + Vincristine + Prednisolone.
7. Tumours often
become resistant to any drug that is used repeatedly due to selection of less
responsive cells. Such selection is favoured if low dose of a single drug is
used.
Several mechanisms of
tumour resistance have been recognized. Mutations altering the target
biomolecule confer specific (to single drug) resistance. An important mechanism
of multidrug resistance is overexpression of MDR 1 gene which increases the
concentration of Pglycoprotein (an efflux transporter) on the surface of cancer
cells, resulting in pumping out of the chemotherapeutic agents, especially
natural products like vinca alkaloids, anthracycline antibiotics, taxanes, etc.
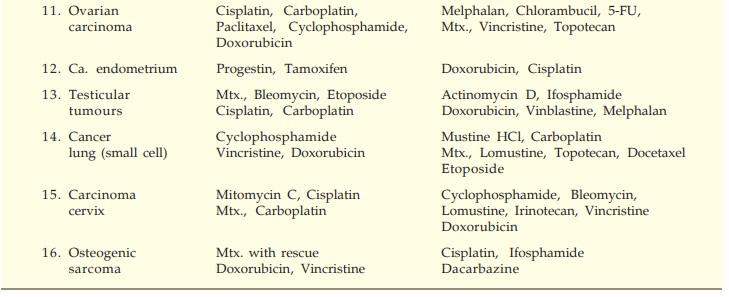
The currently
preferred drugs in chemotherapy-responsive malignancies are listed in Table
62.1.
Toxicity Amelioration
High doses and
intensive regimens are being employed aiming at cure of the malignancy. The associated
toxicity may be ameliorated to some extent by—
1. Toxicity Blocking
Drugs: Folinic acid rescue has
permitted administration of > 100 fold dose of Mtx. It is professed that
normal cells are rescued more than the cancer cells— therapeutic index is
increased.
· Cystitis caused by cyclophosphamide and
ifosphamide can be blocked by systemically administered mesna and by irrigating the bladder with acetylcysteine. Both these are –SH containing compounds that
combine with and detoxify the toxic metabolites in the bladder. Generous fluid
intake and frequent bladder voiding also helps.
· For controlling cytotoxic drug induced
vomiting, ondansetron, a 5HT3
antagonist, has surpassed the efficacy of metoclopramide, which nevertheless is
still used (see Ch. No. 47). Addition
of dexamethasone and/or lorazepam further enhances the protection against
vomiting.
Hyperuricaemia
occurring as a consequence of rapid destruction of bulky tumour masses and
degradation of large amount of purines can be reduced by allopurinol, alkalinization of urine and plenty of fluids.
Corticosteroids also reduce hyperuricemia.
Hypercalcaemia
occurring as a complication of certain malignancies like myeloma, cancer
breast/prostate, etc. may be aggravated by chemotherapy. It is treated by
vigorous hydration and i.v. bisphosphonates (see Ch. No. 24).
Drugs given in pulses
with 2–3 week intervals for normal cells to recover improve the efficacy of
therapy: malignant cells recovering more slowly.
Selective exposure of
tumour to the drug by intraarterial infusion into a limb or head and neck;
intrapleural/intraperitoneal injection— especially for rapidly accumulating
pleural effusion or ascitis; topical application on the lesion—on skin, buccal
mucosa, vagina, etc. may reduce systemic toxicity.
Platelet and/or
granulocyte transfusion after treatment—to prevent bleeding or infection.
Use of biological response modifiers like recombinant GMCSF/GCSF
hastens recovery from cytotoxic drug induced myelosuppression.
Molgramostim (LEUCOMAX 150, 300, 400 μg/vial for s.c./i.v. inj) is a colony
stimulating factor. Injected daily beginning one day after last dose of
myelosuppressant chemotherapy, it hastens recovery of neutrophil count.
Interleukin2 (Il2) is
a cytokine biological response modifier that itself has antitumour property by
amplifying killer Tcell response.
Bone marrow transplantation after treatment with high doses of
myelosuppressant drugs.
Thalidomide
(banned in 1960 for its teratogenic effect) has
anxiolytic, antiemetic, adjuvant analgesic/antipyretic properties and has been
found to counteract cancer associated cachexia. It probably acts by suppressing
TNFα and by modulating IL-2
