Free Energy
| Home | | Biochemistry |Chapter: Biochemistry : Bioenergetics and Oxidative Phosphorylation
The direction and extent to which a chemical reaction proceeds is determined by the degree to which two factors change during the reaction.
FREE ENERGY
The direction and
extent to which a chemical reaction proceeds is determined by the degree to
which two factors change during the reaction. These are enthalpy (∆H, a measure
of the change in heat content of the reactants and products) and entropy (∆S, a
measure of the change in randomness or disorder of reactants and products;
Figure 6.1). Neither of these thermodynamic quantities by itself is sufficient
to determine whether a chemical reaction will proceed spontaneously in the
direction it is written. However, when combined mathematically (see Figure
6.1), enthalpy and entropy can be used to define a third quantity, free energy
(G), which predicts the direction in which a reaction will spontaneously
proceed.
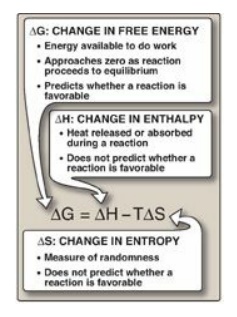
Figure 6.1 Relationship
between changes in free energy (G), enthalpy (H), and entropy (S). T is the
absolute temperature in Kelvin (K): K = oC + 273.
Related Topics
