Filling and Drying
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : The Manufacture And Quality Control Of Immunological Products
As vaccine is required to meet orders, bulk vaccine is distributed into single-dose ampoules or into multidose vials as necessary. Vaccines that are filled as liquids are sealed and capped in their containers, whereas vaccines that are provided as dried preparations are freeze-dried before sealing.
FILLING AND DRYING
As vaccine is required to meet orders, bulk vaccine is distributed into
single-dose ampoules or into multidose vials as necessary. Vaccines that are
filled as liquids are sealed and capped in their containers, whereas vaccines
that are provided as dried preparations are freeze-dried before sealing.
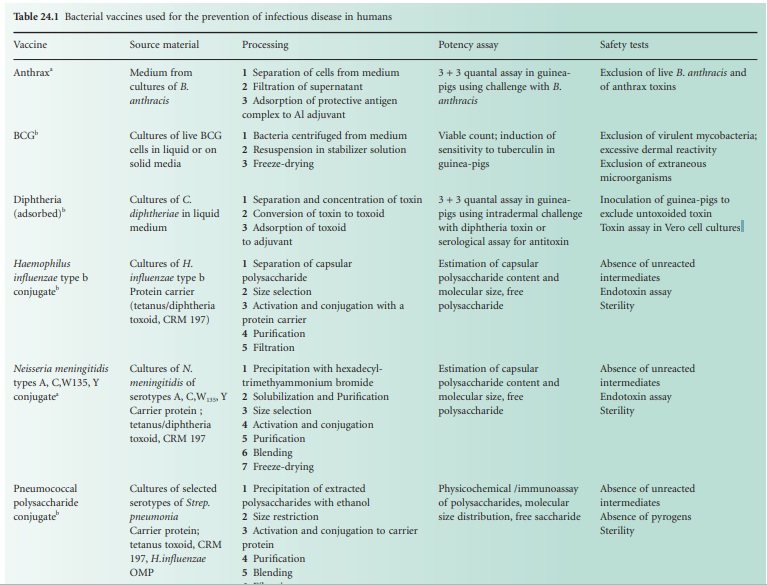
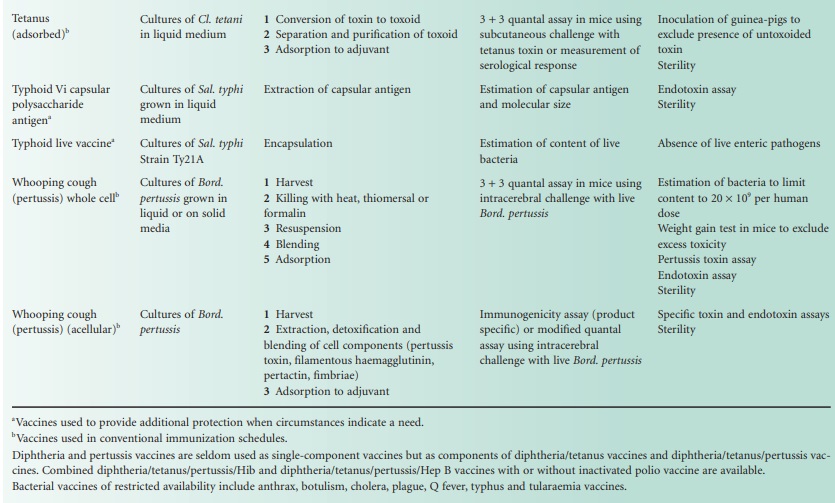
The single-component bacterial vaccines
are listed in Table 24.1.
For each vaccine, notes are provided of the basic material from which the
vaccine is made, the salient production processes and tests for potency and for
safety. The multicomponent vaccines that are made by blending together two or
more of the single-component vaccines are required to meet the potency and
safety requirements for each of the single components that they contain. The
best-known of the combined bacterial vaccines is the adsorbed diphtheria,
tetanus and pertussis vaccine (DTPer/Vac/Ads) that is used to immunize infants,
and the adsorbed diphtheria and tetanus vaccine (DT/Vac/Ads) that is used to
reinforce the immunity of school entrants. The trend is to produce increasingly
complex combinations, and hepta-and octavalent preparations are now available.
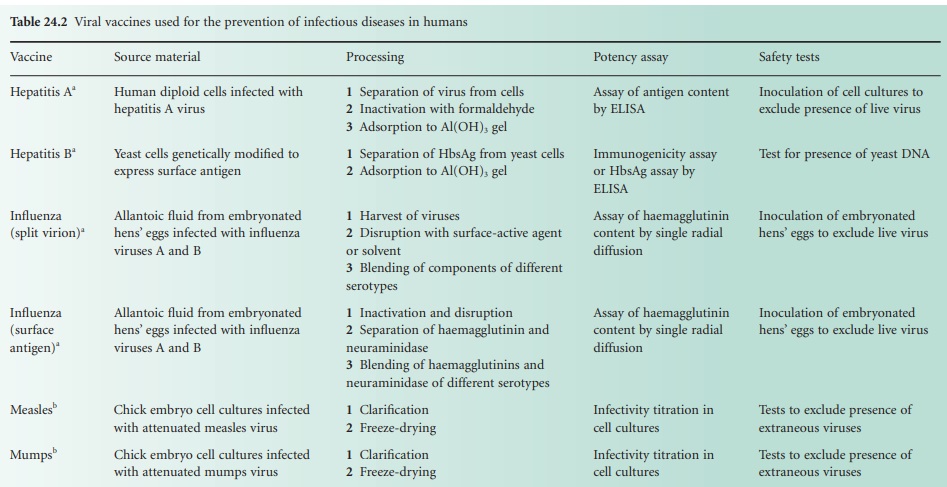
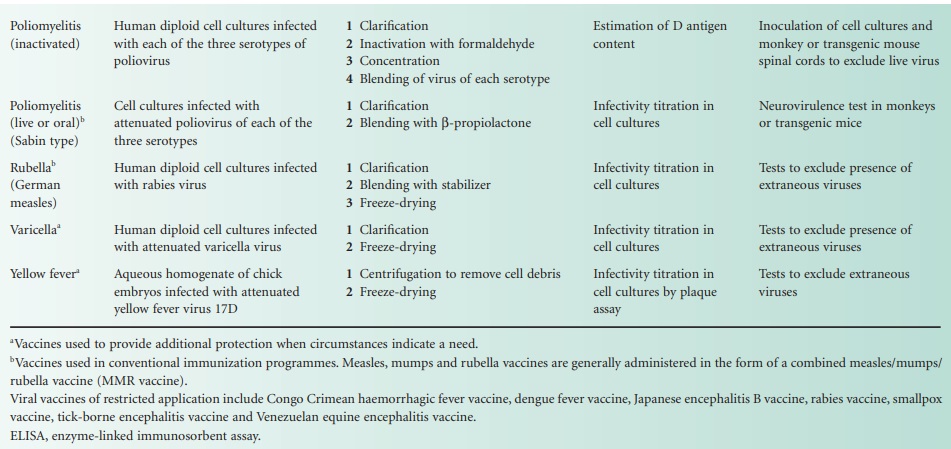
The single-component viral vaccines are listed in Table 24.2, with notes
similar to those
provided with the bacterial vaccines. The only
combined viral vaccine
that is widely
used is the measles, mumps
and rubella vaccine (MMR Vac). In a sense however, both the inactivated (Salk) poliovaccine (Pol/Vac (inactivated)) and the live (Sabin) poliovaccine (Pol/Vac (oral) are combined vaccines in that
they are both
mixtures of virus
of each of the three serotypes
of poliovirus. Influenza vaccines, too, are combined vaccines in that they
usually contain components from several virus strains,
usually from two strains of influenza A and one
strain of influenza B.
Related Topics
