Ferguson Principle
| Home | | Medicinal Chemistry |Chapter: Medicinal Chemistry : Ferguson Principle
The observation that many compounds containing diverse chemical groups exhibit narcotic or anaesthetic action is indicative of the fact that mainly physical rather than chemical properties are involved.
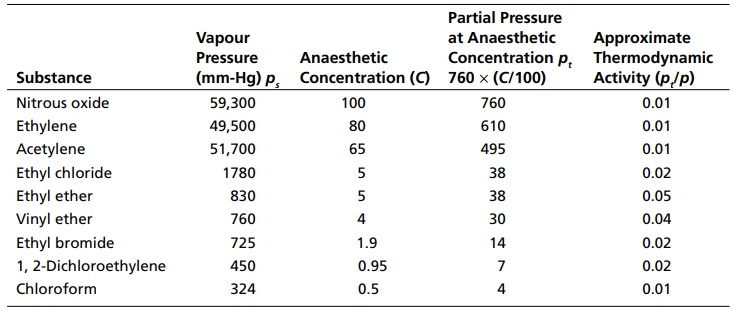
Ferguson Principle INTRODUCTION The observation that many compounds containing diverse chemical groups exhibit narcotic or anaesthetic action is indicative of the fact that mainly physical rather than chemical properties are involved. The fact that narcotic action is attained rapidly and remains at the same level as long as reservoir or critical concentration of the drug is maintained, but quickly disappears when the supply of drug is removed suggests that equilibrium exists between the external phase and the biophase. According to Ferguson, it is unnecessary neither to define the nature of the biophase or the receptor nor to measure the concentration of the drug at this site. If equilibrium conditions exist between the drug in molecular biophase and in extracellular fluids, the tendency to escape from each phase is the same, even though the concentrations in the two phases are different. This tendency is called thermodynamic activity. It is approximately equivalent to the degree of saturation of each phase. Since, the thermodynamic activity is the same in both the biophase and the extracellular phase, measurements made in the extracellular phase, which is measurable, may be directly equated with biophase, which is not possible to be measured. On the basis of the mode of action, drugs are divided into two categories (Table 2.1): structurally nonspecific drugs structurally specific drugs The thermodynamic activity of nonvolatile drugs may be calculated from the expression S/So, where S = molar concentration of the drug and So = solubility of the drug. In the case of volatile drugs, their thermodynamic activity is calculated by using the formula pt/ps. where pt = partial pressure of the substance in solution, pt = 760 × (C/100) ps = saturated vapour pressure C = concentration For example, Saturated vapour pressure of CHCl3 (ps) = 324 Narcotic concentration C = 0.5 Structurally Nonspecific Drugs 1. Their biological action is directly related to the thermodynamic activity 2. Thermodynamic activity value varied from 0.01 to 1 3. High doses are needed for biological activities 4. Chemical structures are different, but they produce similar biological responses 5. Slight modifications in their chemical structure do not result in pronounced changes in biological action Structurally Specific Drugs 1. Their biological action does not depend on the thermodynamic activity 2. Thermodynamic activity value is below 0.01 3. They are effective in low concentrations 4. They have some structural characteristics in common to produce the biological response 5. Slight modifications in their chemical structure may result in substantial changes in biological activity Partial pressure of CHCl3 (pt) = 760 × (C/100) = 760 × (0.5/100) = 760 × 0.005 = 3.8 ∞ 4 Approximate thermodynamic activity = pt /ps = 4/324 = 0.01 The isoanaesthetic concentration of gases and vapours are given in Table 2.2. These findings coined the Ferguson’s principle, which states that ‘substances that are present at the same proportional saturation in a given medium have the same degree of biological action’.Table 2.1 Differences between structurally nonspecific drugs and structurally specific drugs.
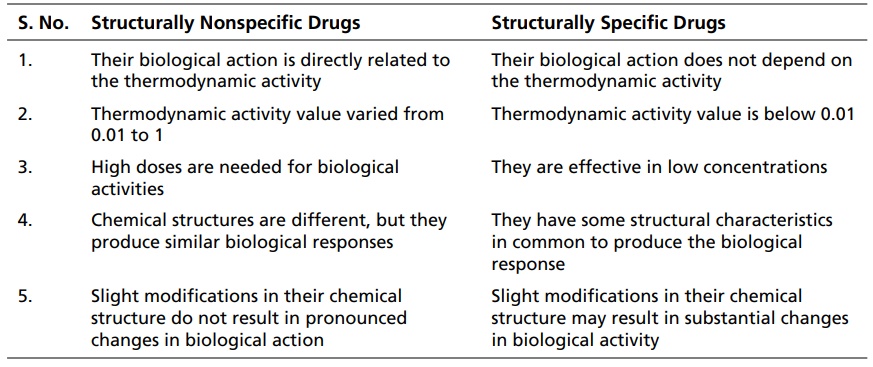
Differences between structurally nonspecific drugs and structurally specific drugs.
Table 2.2 Isoanaesthetic concentration of gases and vapours in man at 37°C.