Evaporation of Water into an Airstream
| Home | | Pharmaceutical Technology |Chapter: Pharmaceutical Engineering: Drying
The evaporation of moisture into a warm airstream, the latter providing the latent heat of evaporation, is a common drying mechanism, although it is not easily adapted to the recovery of the liquid.
EVAPORATION OF WATER INTO AN
AIRSTREAM
The
evaporation of moisture into a warm airstream, the latter providing the latent
heat of evaporation, is a common drying mechanism, although it is not easily
adapted to the recovery of the liquid. We will consider first evaporation from
a liquid surface, which, with the passage of air, falls to the wet bulb
temperature corresponding to the temperature and humidity of the air, as
described in chapter 6. The rate at which water vapor is transferred from the
saturated layer at the surface to the drying stream is described by equation
(4.5) in chapter 4 as:
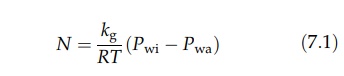
where
Pwi is the partial pressure of the water vapor at the surface and Pwa
is the partial pressure of water vapor in the air. kg is a mass transfer coefficient, and N is the number
of moles of vapor transferred from unit area in unit time. Rewriting this in
terms of the total mass, W,
transferred in unit time from the entire drying surface, A,
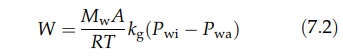
where
Mw is the molecular weight of water vapor, R is the gas constant, and T
is the absolute temperature.
The
mass transfer coefficient, kg,
will itself be a function of the temperature, the air velocity, and its angle
of incidence. A high velocity or angle of incidence diminishes the thickness of
the stationary air layer in contact with the liquid surface and, therefore,
lowers the diffusional resistance.
The
rate of evaporation may also be expressed in terms of the heat transferred
across the laminar film from the drying gases to the surface. This is described
by equation (3.7) in chapter 3 as:
Q
= hA(Ta - Ts) (7:3)
where
Q is the rate of heat transfer, A is
the area of the surface, Ta
and Ts are the
temperatures of the drying air and the surface, respectively, and h is the heat transfer coefficient. The
latter is also a function of air velocity and angle of impingement. If the
latent heat of evaporation is λ,
this affords a mass transfer rate, W,
which is given by
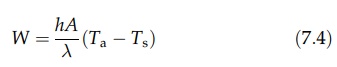
Equilibrium
drying conditions are represented by the equality of equa-tions (7.2) and
(7.4). When these conditions pertain to drying, the surface temperature, Ts,
which is the wet bulb temperature, is normally much lower than the temperature
of the drying gases. This is of great importance in the drying of thermolabile
materials.
If
solids are present in the surface, the rate of evaporation will be modified,
the overall effect depending on the structure of the solids and the moisture
content.
Related Topics
