Equimolecular Counter Diffusion
| Home | | Pharmaceutical Technology |Chapter: Pharmaceutical Engineering: Mass Transfer
Pharmaceutical Engineering : Mass Transfer - Equimolecular Counter Diffusion
EQUIMOLECULAR COUNTERDIFFUSION
If
no bulk flow occurs in the element of length dx, shown in Figure 4.1, the rates of diffusion of the two gases, A
and B, must be equal and opposite, that is,
NA= - NB
The
partial pressure of A changes by dPA
over the distance dx. Similarly, the
partial pressure of B changes by dPB.
Since there is no difference in total pressure across the element (no bulk
flow), dPA/dx must equal -dPB/dx. For an
ideal gas, the partial pressure is related to the molar concentration by the
relation
PAV
= nART
where
nA is the number of moles of gas A
in a volume V. Since the molar
concentration, CA, is equal to nA/V,
PA
= CART
Therefore,
for gas A, equation (4.1) can be written as
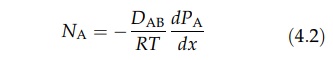
where
DAB is the diffusivity of A in B. Similarly,
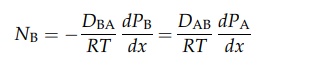
It
therefore follows that DAB = DBA = D. If the partial pressure of A at x1 is PA1 and that
at x2 is PA2, integration of equation (4.2) gives
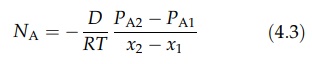
A
similar equation may be derived for the counterdiffusion of gas B.
Related Topics
