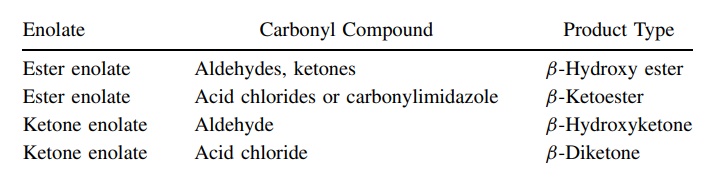
Enolates
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Carbon-Carbon Bond Formation Between Carbon Nucleophiles and Carbon Electrophiles
Enolates are important nucleophiles which react nicely with a variety of carbonyl compounds. In this case, the nucleophilic reactivity of the enolate and the electrophilic reactivity of the carbonyl group are well matched and a wide variety of products can be made.
ENOLATES
Enolates
are important nucleophiles which react nicely with a variety of carbonyl
compounds. In this case, the nucleophilic reactivity of the enolate and the
electrophilic reactivity of the carbonyl group are well matched and a wide
variety of products can be made. The type of enolate (ketone, ester, etc.) and
the type of carbonyl electrophile (aldehyde, ketone, ester, etc.) determine the
structure of the final product. Furthermore these reactions are often named
according to the two partners that are reacted and the type of product produced
from them.
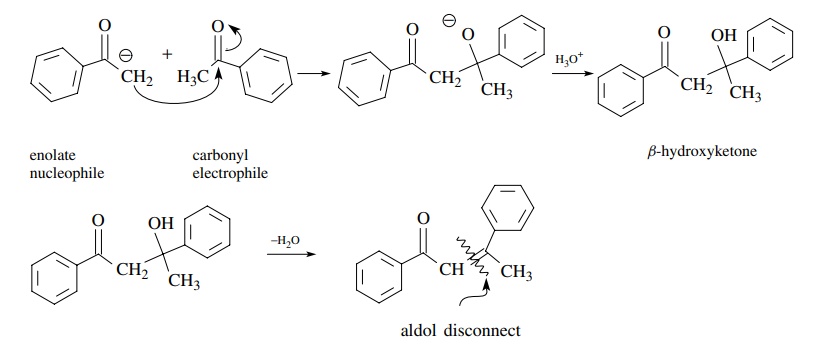
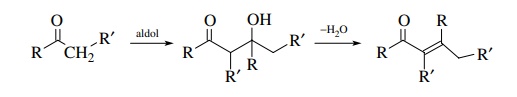
The
aldol condensation is the reaction of an aldehyde or ketone enolate with an
aldehyde or ketone to give a β-hydroxy
aldehyde or ketone. A simple aldol reaction is one in which the enolate
nucleophile is derived from the carbonyl electrophile. Very often the β-hydroxy carbonyl product dehydrates to
give an ,β-unsaturated carbonyl
compound; however, the aldol nature of the dehy-dration product can be
discerned by disconnection of the double bond of the unsaturated product.
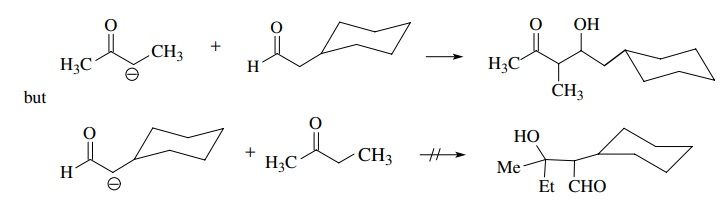
If
the enolate nucleophile is derived from an aldehyde or ketone different than
the carbonyl electrophile, a crossed-aldol condensation results. Normally best
success is achieved if the carbonyl electrophile employed for the crossed-aldol
condensation is more reactive than the carbonyl electrophile from which the
enolate is derived. For example, ketone enolates react with aldehydes
effectively, but aldehyde enolates do not give the crossed aldol with most
ketones but self-condense instead.
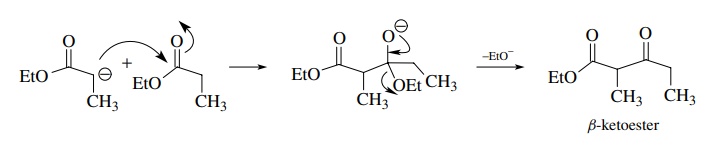
The
Claisen condensation is the reaction of the enolate of an ester with an ester
electrophile. The product is a β-keto
ester since the tetrahedral intermediate collapses by expulsion of an alkoxide.
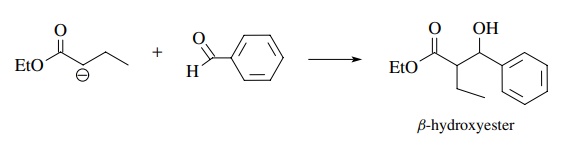
A
crossed Claisen is the reaction of an ester enolate with an aldehyde or ketone
to produce a β-hydroxy ester. This
works well because aldehydes and ketones are more reactive electrophiles than
esters; thus the ester enolate reacts faster with the aldehyde or ketone than it
condenses with itself, avoiding product mix-tures. Moreover, the aldehyde or
ketone should not have α hydrogens so
that proton transfer to the more basic ester enolate is avoided. This would
lead to the formation of an aldehyde or ketone enolate in the mixture, and an
aldol reaction would be a major competing reaction.
For the same reason it is generally not feasible to carry out a crossed-Claisen reaction between the enolate of one ester and a second ester which has α protons. This is due to the fact that if nucleophilic addition to the carbonyl group is not fast, proton exchange can occur, giving a mixture of enolates and thus a mixture of products.
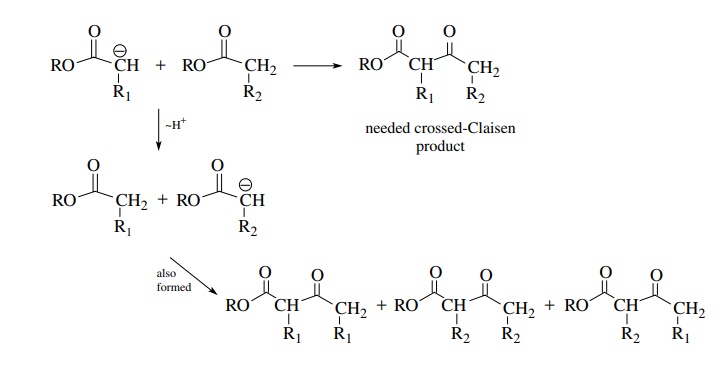
There
are many other named reactions that follow the same general features but differ
as to the type of enolate or the carbon electrophile. These include the
Reformatski reaction, the Darzens reaction, and the Dieckmann ring closure.
They were in widespread use for many years and were named as a convenient way to
characterize the reactants employed and type of product which results. The
reason that there are so many variations on the same theme is that control of
the reaction products depends on the ability to generate a particular enolate
nucleophile and react it with a particular carbonyl electrophile. In earlier
times alkoxide bases were the strongest bases routinely available to synthetic
chemists. Since alkoxides have pKa
= 15–19 while protons
α to carbonyl groups have pKa = 20–25, the reaction of an alkoxide
base with a carbonyl compound produces only a small amount of the enolate at
equilibrium, and it is produced in the presence of the unreacted carbonyl
compound, which is an electrophile. For simple aldol and Claisen reactions,
this is the ideal situation for self-condensation.
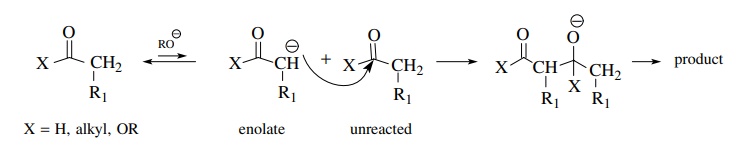
If,
however, it is necessary to generate a crossed product by the reaction of an
enolate derived from one carbonyl compound with a second carbonyl compound as
the electrophile, things can go bad rapidly. Because both carbonyl groups must
be present in solution at the same time and each can form enolates to some
extent, there can be four possible products from the various combinations of
enolates and carbonyl compounds. This problem was illustrated for the
crossed-Claisen condensation above. The number of products can be minimized if
one carbonyl component lacks α
protons and cannot form an enolate and is also a more reactive electrophile
than the second carbonyl component. If these conditions are met, then crossed
condensations can be carried out successfully using alkoxide bases. Many of the
named reactions were developed so that product mixtures could be avoided.
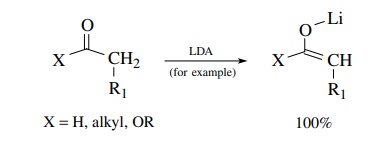
Today
reactions of enolates are usually carried out much differently by uti-lizing
very strong, nonnucleophilic bases for generating the enolate nucleophile.
Instead of having only small equilibrium concentrations of an enolate produced
in solution, the use of strong, nonnucleophilic bases like LDA, KHMDS, and KH
that have pKa’s >35 permits carbonyl compounds, whose
α protons have pKa’s of 20–25, to be converted completely to enolate
anions. Doing so completely converts the carbonyl compound into a nucleophile
which cannot condense with itself and is stable in solution. This enolate can
then be reacted with a second carbonyl compound in a subsequent step to give
product:
Step
1
Step
2
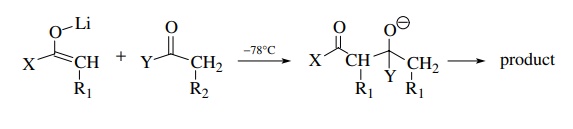
As
long as nucleophilic addition of the preformed enolate to the second carbonyl
component is rapid and the carbonyl electrophile is added after the enolate is formed, the product is predictable and is not
a mixture. The rule of thumb to ensure success is that the carbonyl
electrophile should be more reactive than the carbonyl compound from which the
enolate is derived. If this condition is met, the carbonyl electrophile can
have α protons and the structural
possibilities are increased tremendously. Typical enolate–carbonyl pairs that
have been condensed by this methodology include the following:
Acetylides
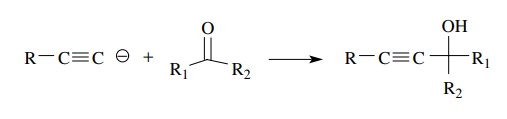
can also react as nucleophiles toward aldehydes and ketones to give propargylic
alcohols, which provides a simple way to install the triple bond in molecules.
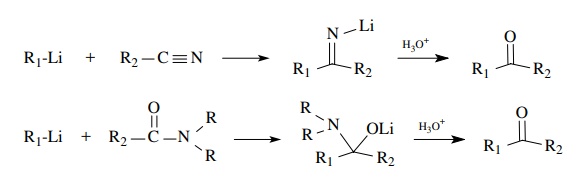
Esters,
amides, and nitriles are relatively weak electrophiles. They react slug-gishly
or not at all with enolates. Esters are more electrophilic than amides and
nitriles and react readily with carbanionic-type reagents such as
organolithiums or Grignard reagents. As seen previously, two equivalents of the
organometallics are added and tertiary alcohols are produced. Tertiary amides
and nitriles react with organolithiums (but not Grignard reagents) to give
ketones after hydrolytic workup. A single nucleophilic addition occurs to give
an anionic intermediate which is stable to further nucleophilic addition. The
oxidation level is that of a ketone which is unmasked upon hydrolysis.
Carbonyl
electrophiles are obviously a very important group of electrophiles that react
successfully with a spectrum of carbon nucleophiles. Among carbonyl
electrophiles, however, large differences in reactivity are observed. Acid
chlorides are very reactive electrophiles whereas esters and amides are much
weaker and fail to react with several classes of carbon nucleophiles. Aldehydes
and ketones are probably the most widely utilized groups of carbonyl
electrophiles and exhibit moderate electrophilic reactivity. No matter what
carbonyl electrophile is used, however, it reacts by nucleophilic addition to
the carbonyl carbon to produce a tetrahedral intermediate. The ultimate
reaction product reflects subsequent chem-istry of the tetrahedral
intermediate.
While
the use of strong bases has changed the way in which many condensation
reactions are carried out, it is important to remember the types of products
that are produced from them. Recall that the aldol condensation yields β-hydroxy aldehydes or ketones which are
easily dehydrated to α,β-unsaturated aldehydes or ketones.
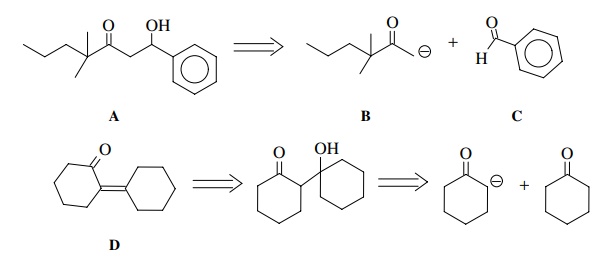
It
is thus possible to look at a molecule such as A below and recognize that it is a β-hydroxy ketone and thus could be formed in a crossed-aldol
reaction between enolate B and
aldehyde C. Likewise D could potentially be produced by
dehydration of the aldol product of cyclohexanone
In
addition to these intermolecular processes, intramolecular versions of the
Claisen (Dieckmann) reaction and the mixed Claisen and the aldol reaction
(Robinson annulation) are also well known. In all cases the same structural
classes of products are formed.
Related Topics
