Emulsification
| Home | | Pharmaceutical Drugs and Dosage | | Pharmaceutical Industrial Management |Chapter: Pharmaceutical Drugs and Dosage: Dosage forms - Emulsions
Emulsification can be facilitated by the following three mechanisms.
Emulsification
Mechanisms of emulsification
Emulsification
can be facilitated by the following three mechanisms:
1.
Reduction of interfacial tension.
2.
Formation of a monomolecular film at the interface that
physically inhibits coalescence of dispersed-phase granules.
3.
Changing the zeta potential of the dispersed phase.
Emulsifying
agents can be surfactants, hydrophilic colloids, or finely divided solid
particles. Table 17.2 lists some of the
commonly used emulsi-fying agents.
Table 17.2 Typical emulsifying agents
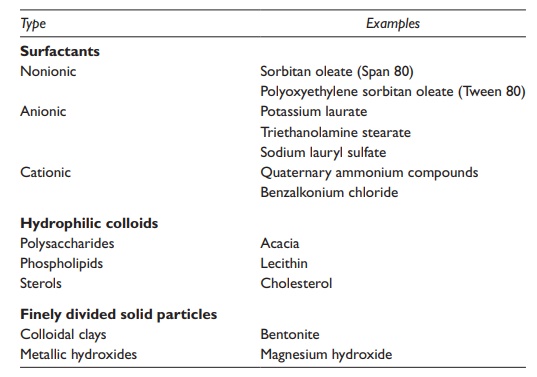
Surfactants
Surfactants are amphiphilic molecules, which contain both a polar hydro-philic region and a nonpolar hydrophobic region. Depending on the func-tional groups and relative surface areas of the two regions, surfactants could have a range of hydrophilic and hydrophobic properties. The use of predominantly hydrophilic emulsifying agent leads to the formation of an o/w emulsion since it has stronger and/or greater area of interac-tion with the aqueous than the oily phase. Conversely, the use of a pre-dominantly hydrophobic emulsifying agent tends to form a w/o emulsion because it has stronger and/or greater area of interaction with the oily than the aqueous phase.
Since
the same surfactant molecule has attractive interactions with both the oily and
aqueous phase, surfactants get adsorbed at the oil–water inter-faces to form monomolecular films. This results in a
decrease in interfacial tension and physical barrier to collision of
dispersed-phase globules. Often, simultaneous use of a predominantly
hydrophilic with a predominantly hydrophobic surfactant is used to form more
stable emulsions, due to the close packing, strength, and flexibility of the
interfacial layer. In addition, the use of ionized surfactants can impact the zeta potential of the dispersed phase.
This mechanism can further improve emulsion stability by increas-ing or
decreasing electrostatic repulsive forces and facilitating flocculation.
Ionic and nonionic surfactants
Surfactants
could be anionic (containing anionic, or acidic, functional groups, such as
sulfates and carboxylates that become negatively charged at solution pH higher
than their pKa), cationic
(containing cationic, or basic, functional groups, such as amines that become
positively charged at solu-tion pH higher than their pKa), amphoteric (containing both anionic and cationic
functional groups, with a propensity to become either or both posi-tively and
negatively charged, depending on the pH), and nonionic (without any ionizable
functional groups, such as alcohols). In addition, surfactants that bear a
quaternary ammonium ion bear a permanent positive charge.
Ionized
surfactants tend to have strong and specific interactions with a variety of
molecules and are, consequently, more toxic than nonionic sur-factants.
Nonionic surfactants are less sensitive to variations in the electro-lyte
content and pH of the formulation. Nonionic surfactants, such as the alkyl or
aryl polyoxyethylene ethers, sorbitan polyoxyethylene derivatives, and sorbitan
are widely used for producing stable emulsions.
Hydrophile-lipophile balance value
The
relative hydrophobicity and hydrophilicity of a surfactant is indicated by its
hydrophile–lipophile balance (HLB) value. A typical HLB value scale ranges from
0 to 20. An emulsifying agent with high HLB (~9–12) is pref-erentially soluble
in water and favors the formation of an o/w emulsion. Conversely, surfactants
with low HLB value (~3–6) are preferentially oil soluble and tend to form w/o
emulsions.
The
HLB system assumes the hydrophilic contribution of the surfac-tant from
ionizable and water-miscible functional groups, such as poly-hydric alcohols,
ethylene oxide group, fatty acid, or fatty alcohol groups. The hydrophilic
portion of a molecule is calculated on a molecular weight basis and divided by
5 to arrive at the HLB value. In general, surfactants with an HLB value of 1–3
can be used for mixing oils, 4–6 for making w/o emulsions, 7–9 for wetting
powders into oils, 7–10 for making self-emulsifying systems, 8–16 for making
o/w emulsions, 13–15 for making detergents, and 13–18 for making
self-microemulsifying systems.
Hydrophilic colloids
Hydrophilic
colloids are polymeric materials that bear several electronega-tive atoms, such
as oxygen and nitrogen, thus having strong hydrophilicity through dipole–dipole
interactions and hydrogen bond formation. Several hydrophilic colloids, such as
gelatin, casein, acacia, cellulose derivatives, and alginates, are used as
emulsifying agents. Hydrophilic colloids are used for formation of o/w
emulsions since the films are hydrophilic. These mate-rials adsorb at the
oil–water interface and form multilayer
films around the dispersed droplets of oil in an o/w emulsion. Most
cellulose derivatives are not charged, but can sterically stabilize the
systems.
Hydrated
hydrophilic colloids differ from surfactants because they do not cause an
appreciable lowering in interfacial tension. They stabilize emul-sions by the
formation of multilayer films that are strong and resist coales-cence. In
addition, they increase the viscosity of the dispersion medium.
Finely divided solid particles
Finely
divided solid particles that are wetted to some degree by both oil and water
can act as emulsifying agents by concentrating at the interface, where they
produce a film of particles around the dispersed droplets and act as a physical
barrier to coalescence. Finely divided solid particles that are predominantly
wetted by water form o/w emulsions, whereas those that are predominantly wetted
by oil form w/o emulsions. Examples include bentonite, magnesium hydroxide, and
aluminum hydroxide.
