Electrolyte Balance
| Home | | Anatomy and Physiology | | Anatomy and Physiology Health Education (APHE) |Chapter: Anatomy and Physiology for Health Professionals: Fluid, Electrolyte, and Acid Base Balance
When the quantities of electrolytes the body gains equal those it loses, electrolyte balance exists. This is also maintained by homeostasis.
Electrolyte
Balance
When the quantities of electrolytes the body gains equal
those it loses, electrolyte
balance exists. This is also maintained by homeostasis. The most
import-ant electrolytes needed for cellular functions are sodium, potassium,
calcium, magnesium, chloride, sulfate, phosphate, bicarbonate, and hydrogen
ions. These are mostly provided in food but also are pres-ent in water and
other beverages and as byproducts of metabolic reactions. Of all the
electrolytes, sodium imbalance is most significant. A severe deficiency of
electrolytes may produce a desire to eat salty foods known as salt craving. Salts help to control
fluid move-ment in the body and provide needed minerals for excitability,
membrane permeability, and secretory activities. Potassium and calcium are also
among the most important electrolytes.
More electrolytes are lost by sweating on warm days and
during strenuous exercise. Additional amounts are lost in the feces, but the
greatest elec-trolyte output occurs because of kidney function and urine
production. The kidneys control electrolyte out-put to maintain balance.
Positive ions such as calcium, potassium, and sodium are essential for
maintenance of cell membrane potential, muscle fiber contrac-tion, and nerve
impulse conduction. Nearly 90% of positively charged ions in the extracellular
fluids are sodium ions, which are regulated by the kidneys and the hormone
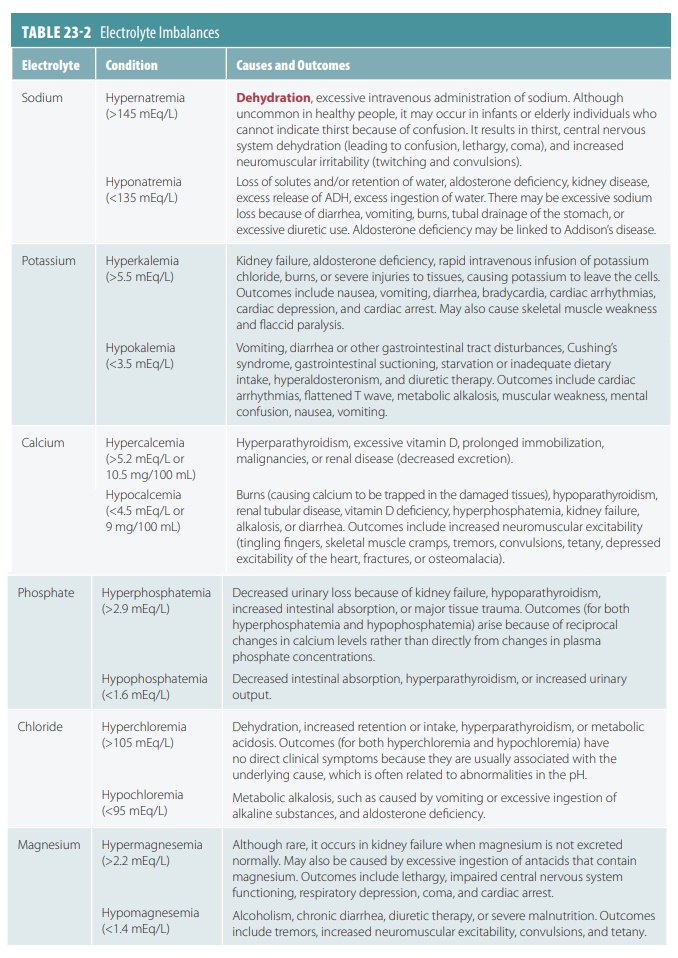
aldosterone. Electrolyte imbalances are summarized in TABLE 23-2.
Sodium Balance
Salts primarily enter the body in foods and fluids, but
lesser amounts are due to metabolic activity. During catabolism of bone matrix
and nucleic acids, phosphates are liberated. It is not difficult for us to
obtain the amounts of electrolytes we need, how-ever. Most Americans eat much
more sodium than they actually need. Natural foods contain plenty of sodium,
whereas processed foods contain too much sodium. Table salt or sodium chloride is used to excess.
Salts are mostly lost from the body via sweating, vomiting,
and in the urine and feces. Sweat is usually hypotonic, but large amounts of
salt can be lost when sweating becomes profuse. Large losses of salt in vomit
or feces may be linked to disorders of the gastrointes-tinal tract. To balance salts
in the body, the kidneys must be healthy.
Potassium Balance
The intracellular fluid contains nearly 98% of the body’s
potassium. This electrolyte diffuses out of the cellular cytoplasm into the
extracellular fluid and therefore requires the cells to expend energy to
recover its ions. The potassium ion concentration in the extracellular fluid is
based on a balance between the rate at which the ions are gained across the
diges-tive epithelium and the rate at which they are lost into the urine. The
actions of the ion pumps in the distal parts of nephrons and the collection
system regulate potassium loss in the urine. When a sodium ion is reabsorbed
from the tubular fluid, there is usually an exchange between it and a cation,
most commonly potassium, from the peritubular fluid.
Normally, between 50 and 150 mEq of urinary potassium ions
are lost, whereas the same amount is absorbed across the digestive epithelium.
There is only a small amount of potassium lost in the perspi-ration and feces.
In the extracellular fluid, potassium ion concentration is controlled by
regulating active secretion rates along the distal convoluted tubule and
nephron collecting system.
Three factors relate to how the rate of tubular secretion of
potassium ions varies: changes in the potassium ion concentration of the
extracellular fluid, changes in pH, and aldosterone levels. Basically, the
higher the concentration of potassium in the extracel-lular fluid, the higher
the rate of secretion. When pH falls in the extracellular fluid, the pH of the
peritubu-lar fluid also falls. There is then a decline in the rate of potassium
secretion. This is because hydrogen ions, not potassium ions, are secreted as
part of an exchange with sodium ions in the tubular fluid.
Aldosterone greatly affects the rate at which potassium ions
are lost in the urine. This results from the ion pumps being sensitive to
aldosterone and therefore reabsorbing sodium ions from the filtrate, exchanged
for potassium ions from the peritubular fluid. Angiotensin II stimulates
aldosterone secre-tion as part of blood volume regulation. Aldosterone
secretion is also directly stimulated by high plasma potassium ion
concentrations. The ways aldosterone influences the amounts of conserved sodium
and the amounts of potassium excreted via the urine are closely related. Once
plasma concentrations of potas-sium fall below 3.5 mEq/L hypokalemia develops, with extensive muscular weakness being
followed by paralysis. Hypokalemia may cause death by affecting normal cardiac
function.
Calcium Balance
There is more calcium in the body than any other mineral,
and 99% of body calcium is deposited in the skeleton. This makes up 1 to 2 kg,
or 2.2–4.4 pounds, of body calcium. Calcium is vital for controlling muscular
and neural activities, for blood clotting, for forming the crystalline
components of bones, as a cofactor for enzymatic reactions, and because of its second messenger functions. Calcium
homeostasis is maintained in the
extracellular fluid by parathyroid hormone and calcitriol but also by
calcitonin to a smaller degree. Calcium ion concentrations are raised by
parathyroid hormone and calcitriol, whereas calci-tonin opposes their actions.
Calcitriol is produced by the kidneys.
Although a small amount of calcium is lost every day in the bile,
only tiny amounts are lost via the urine or feces. Therefore, an adult must
absorb only 0.8–1.2 g/day of calcium, which is only approx-imately 0.03% of the
amount of calcium stored in the skeleton. Calcium absorption is stimulated by
parathyroid hormone and calcitriol. It is absorbed in the digestive tract and
reabsorbed in the distal convoluted tubule.
Hypercalcemia
is
present when the extracel-lular fluid calcium ion concentration is higher than
5.3 mEq/L. In adults, it is usually caused by hyperparathyroidism, which is
over-secretion of parathyroid
hormone. Additional causes include malignant cancers of the breast, kidneys,
bone marrow, or lungs. Excessive use of supplements containing calcium or
vitamin D may also cause hypercalcemia. Hypercalcemia is considered severe when
calcium ion concentration exceeds 12–13 mEq/L in the extracel-lular fluid.
Signs and symptoms include confusion, fatigue, calcification of soft tissues
such as the kidneys, and cardiac arrhythmias.
The opposite condition is hypocalcemia, in which there is a calcium ion concentration under
4.3 mEq/L. Much less common than hypercalcemia, this is usually caused by hypoparathyroidism, which is
under-secretion of parathyroid hormone, chronic renal failure, or vitamin D
deficiency. Signs and symptoms include osteoporosis, weak heartbeat, muscle
spasms that may be accompanied by generalized convulsions, and cardiac
arrhythmias.
Phosphate Balance
Phosphate
ions are essential for the mineralization of bones. The mineral salts of the skeleton store approximately
740 g of phosphate ions. Phosphate most significantly affects the intracellular
fluid, where it helps to activate enzymes, form high-energy compounds, and
synthesize nucleic acids. In the plasma the normal concentration of phosphate
ions is 1.8–3.0 mEq/L. It is reabsorbed from the tubular fluid in the proximal
convoluted tubule. This reabsorption is stimulated by calcitriol. Via the urine
and feces, approximately 30–45 mEq, or 0.8–1.2 g of phosphate, is lost every
day.
Chloride Balance
Chloride
ions are the most common ions found in the extracellular fluid, with normal plasma concentrations between 100
and 108 mEq/L. Chloride is usually very low in the intracellular fluid,
approximately 3 mEq/L. Chloride is absorbed across the digestive tract along
with sodium. In the renal tubules, chloride and sodium ions are absorbed by
several carrier proteins. Very little loss of chloride ions occurs via the
urine and perspiration. Therefore, only 48–146 mEq, or 1.7–5.1 g/day, are
required to maintain a chloride ion balance.
1. Define
the terms electrolytes, water balance, and electrolyte balance.
2. List
the causes and symptoms of dehydration.
3.
Describe how salts are lost and gained by the body.
Related Topics