Digestion of Dietary Carbohydrates
| Home | | Biochemistry |Chapter: Biochemistry : Introduction to Carbohydrates
The principal sites of dietary carbohydrate digestion are the mouth and intestinal lumen. This digestion is rapid and is catalyzed by enzymes known as glycoside hydrolases (glycosidases) that hydrolyze glycosidic bonds.
DIGESTION OF DIETARY CARBOHYDRATES
The principal sites of
dietary carbohydrate digestion are the mouth and intestinal lumen. This
digestion is rapid and is catalyzed by enzymes known as glycoside hydrolases
(glycosidases) that hydrolyze glycosidic bonds (Figure 7.8 ). Because there is
little monosaccharide present in diets of mixed animal and plant origin, the
enzymes are primarily endoglycosidases that hydrolyze polysaccharides and
oliosaccharides, and disaccharidases that hydrolyse tri- and disaccharides into
their reducing sugar components. Glycosidases are usually specific for the
structure and configuration of the glycosyl residue to be removed as well as
for the type of bond to be broken. The final products of carbohydrate digestion
are the monosaccharides, glucose, galactose, and fructose that are absorbed by
cells of the small intestine.
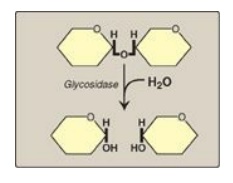
Figure 7.8 Hydrolysis of a glycosidic bond.
A. Salivary a-amylase
The major dietary polysaccharides are of plant (starch, composed of amylose and amylopectin) and animal (glycogen) origin. During mastication, salivary α-amylase acts briefly on dietary starch and glycogen, hydrolyzing random α(1→4) bonds. [Note: There are both α(1→4)- and β(1→4)-endoglucosidases in nature, but humans do not produce the latter. Therefore, we are unable to digest cellulose, a carbohydrate of plant origin containing β(1→4) glycosidic bonds between glucose residues.] Because branched amylopectin and glycogen also contain α(1→6) bonds, which α-amylase cannot hydrolyze, the digest resulting from its action contains a mixture of short, branched and unbranched oligosaccharides known as dextrins (Figure 7.9 ). [Note: Disaccharides are also present as they, too, are resistant to amylase.] Carbohydrate digestion halts temporarily in the stomach, because the high acidity inactivates salivary α-amylase.
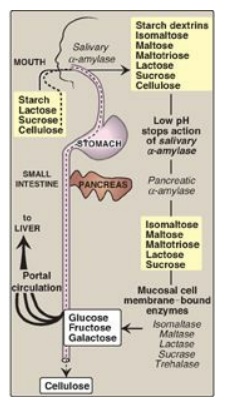
Figure 7.9 Digestion of carbohydrates. [Note: Indigestible cellulose enters the colon and is excreted.]
B. Pancreatic a-amylase
When the acidic stomach
contents reach the small intestine, they are neutralized by bicarbonate
secreted by the pancreas, and pancreatic α-amylase continues the process of
starch digestion.
C. Intestinal disaccharidases
The final digestive
processes occur primarily at the mucosal lining of the upper jejunum and include
the action of several disaccharidases (see Figure 7.9 ). For example,
isomaltase cleaves the α(1→6) bond in isomaltose, and maltase cleaves the
α(1→4) bond in maltose and maltotriose, each producing glucose. Sucrase cleaves
the α(1→2) bond in sucrose, producing glucose and fructose, and lactase
(β-galactosidase) cleaves the β(1→4) bond in lactose, producing galactose and
glucose. [Note: The substrates for isomaltase are broader than its name
suggests, and it hydrolyzes the majority of maltose.] Trehalose, an α(1→1)
disaccharide of glucose found in mushrooms and other fungi is cleaved by
trehalase. These enzymes are transmembrane proteins of the brush border on the
luminal surface of the intestinal mucosal cells.
Sucrase and isomaltase are enzymic activities of a single protein (SI) which is cleaved into two functional subunits that remain associated in the cell membrane, forming the sucrase-isomaltase complex. In contrast, maltase is one of two enzymic activities of a single membrane protein maltase-glucoamylase (MGA) that does not get cleaved. Its second enzymic activity, glucoamylase, cleaves a(1→4) glycosidic bonds in dextrins.
D. Intestinal absorption of monosaccharides
The duodenum and upper
jejunum absorb the bulk of the monosaccharide products of digestion. However,
different sugars have different mechanisms of absorption ( Figure 7.10). For
example, galactose and glucose are transported into the mucosal cells by an
active, energy-dependent process that requires a concurrent uptake of sodium
ions, and the transport protein is the sodium-dependent glucose cotransporter 1
(SGLT-1). Fructose utilizes an energy- and sodium-independent monosaccharide
transporter (GLUT-5) for its absorption. All three monosaccharides are
transported from the intestinal mucosal cell into the portal circulation by yet
another transporter, GLUT-2. (See : for a discussion of these transporters.)
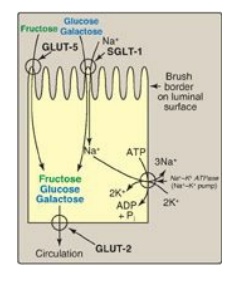
Figure 7.10 Digestion of carbohydrates. [Note: Indigestible cellulose enters the colon and is excreted.]
E. Abnormal degradation of disaccharides
The overall process of carbohydrate digestion and absorption is so efficient in healthy individuals that ordinarily all digestible dietary carbohydrate is absorbed by the time the ingested material reaches the lower jejunum. However, because only monosaccharides are absorbed, any deficiency (genetic or acquired) in a specific disaccharidase activity of the intestinal mucosa causes the passage of undigested carbohydrate into the large intestine. As a consequence of the presence of this osmotically active material, water is drawn from the mucosa into the large intestine, causing osmotic diarrhea. This is reinforced by the bacterial fermentation of the remaining carbohydrate to two- and three-carbon compounds (which are also osmotically active) plus large volumes of CO2 and H2 gas, causing abdominal cramps, diarrhea, and flatulence.
1. Digestive enzyme deficiencies: Genetic deficiencies of the
individual disaccharidases result in disaccharide intolerance. Alterations in
disaccharide degradation can also be caused by a variety of intestinal
diseases, malnutrition, and drugs that injure the mucosa of the small
intestine. For example, brush border enzymes are rapidly lost in normal
individuals with severe diarrhea, causing a temporary, acquired enzyme
deficiency. Therefore, patients suffering or recovering from such a disorder
cannot drink or eat significant amounts of dairy products or sucrose without
exacerbating the diarrhea.
2. Lactose intolerance: More than 70% of the world’s
adults arelactose intolerant (Figure 7.11). This is particularly manifested in
certain populations. For example, up to 90% of adults of African or Asian descent
are lactase-deficient and, therefore, are less able to metabolize lactose than
individuals of Northern European origin. The age-dependent loss of lactase
activity represents a reduction in the amount of enzyme produced. It is thought
to be caused by small variations in the DNA sequence of a region on chromosome
2 that controls expression of the gene for lactase, also on chromosome 2.
Treatment for this disorder is to reduce consumption of milk and eat yogurts
and some cheeses (bacterial action and aging process decrease lactose content)
as well as green vegetables, such as broccoli, to ensure adequate calcium
intake; to use lactase-treated products; or to take lactase in pill form prior
to eating. [Note: Because the loss of lactase is the norm for most of the
world’s adults, use of the term “adult hypolactasia” for lactose intolerance is
becoming more common.] Rare cases of congenital lactase deficiency are known.
3. Congenital sucrase-isomaltase deficiency: This autosomal recessive disorder
results in an intolerance of ingested sucrose. Congenital sucrase-isomaltase
deficiency has a prevalence of 0.02% in individuals of European descent and
appears to be much more common in the Inuit people of Greenland and Canada.
Treatment includes the dietary restriction of sucrose and enzyme replacement
therapy.
4. Diagnosis: Identification of a specific enzyme deficiency can
be obtained by performing oral tolerance tests with the individual
disaccharides. Measurement of hydrogen gas in the breath is a reliable test for
determining the amount of ingested carbohydrate not absorbed by the body, but
which is metabolized instead by the intestinal flora (see Figure 7.11).
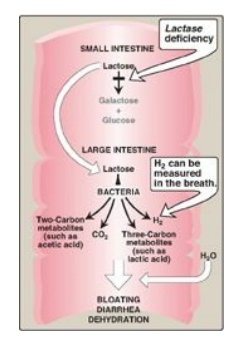
Figure 7.11 Abnormal lactose
metabolism. H2 = hydrogen gas.
Related Topics
