Diels-Alder Reaction
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Planning Organic Syntheses
Another approach for the construction of rings is to use reactions which start with two acyclic compounds and produce cyclic products. There are many of these processes, but the most used and most useful is the Diels–Alder reaction.
DIELS-ALDER REACTION
Another
approach for the construction of rings is to use reactions which start with two
acyclic compounds and produce cyclic products. There are many of these
processes, but the most used and most useful is the Diels–Alder reaction. This
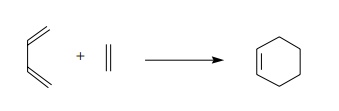
is a reaction between a diene and an olefin to give a new six-membered ring. It
is also termed a 4 +
2 cycloaddition because one partner (the diene) containing four π electrons adds to a two-electron
fragment (the olefin) containing two π
electrons to yield a ring.
The
Diels–Alder reaction is one type of a much larger class of ring-forming
reactions termed cycloaddition reactions.
In most instances, new carbon–carbon bonds are formed during cycloaddition
processes. Such reactions are neither polar reactions (although the reactants
might be polar molecules) nor free-radical reac-tions. Rather cycloadditions
are usually concerted reactions in which all bond making and bond breaking are
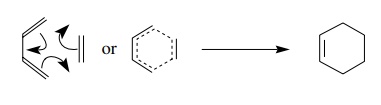
taking place at the same time. Such reactions have been described as having a
“no-mechanism mechanism.” Curved arrows can be used for keeping track of
electrons, but they are by no means of mechanistic significance. Alternatively
dotted lines are sometimes used to describe the transi-tion state, implying
that all bonds are being made and broken simultaneously. No matter which
mechanistic formalism is used to describe the process, the product contains a
six-membered ring which has a double bond.
Concerted
cycloaddition reactions result from the interaction between π sys-tems in two different molecules.
The π system in one molecule reacts
with the π system in a second molecule to produce new bonds. Now since both the
diene and the olefin are closed-shell, ground-state molecules, the first
question that comes to mind is “How do such systems react?”
The
formation of new bonds in the Diels–Alder reaction requires that the π electrons in the individual diene and
olefin π systems become reorganized
and shared in the new bonding pattern of the cyclic product. It follows that
for this bonding change to occur, the two π
systems must overlap so that electrons can move into new orbitals. The most
straightforward way the needed orbital overlap can occur is for one π system to function as an electron
donor and the other π system to function as an electron acceptor. Therefore the
bonding changes in the Diels–Alder reaction result from a donor–acceptor
interaction between the diene and olefin π
systems.
It
is well known that π electrons are
less tightly bound than σ electrons,
and consequently they can readily be donated to a variety of electrophiles
(e.g., bromine, protons, mercuric ion), so it is easy to imagine a π system acting as an electron donor. In
contrast, π systems, being electron
rich, are not often thought of as electron acceptors (which would make them
even more electron rich).
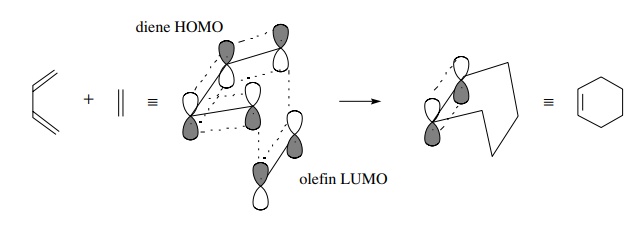
For
a π system to function as an electron
acceptor, it must have unfilled orbitals available to accept electrons. In the
case of olefins or dienes those are π∗ antibonding
molecular orbitals. Thus interaction of the HOMO of one π system with the LUMO of a second π system produces a donor–acceptor pair (HOMO donating to LUMO)
enabling electrons to be transferred from one π system to another with resulting bond formation.
In
picture form this can be drawn as
Related Topics
