Conjugation With Glutathione and Mercapturic Acid Formation
| Home | | Biopharmaceutics and Pharmacokinetics |Chapter: Biopharmaceutics and Pharmacokinetics : Biotransformation of Drugs
Glutathione ( -glutamyl cysteinyl glycine or GSH) is a tripeptide with a strongly nucleophilic character due to the presence of a -SH (thiol) group in its structure.
PHASE II REACTION
CONJUGATION WITH GLUTATHIONE AND MERCAPTURIC ACID FORMATION
Glutathione ( γ-glutamyl cysteinyl glycine or GSH) is a tripeptide with a strongly nucleophilic character due to the presence of a -SH (thiol) group in its structure.
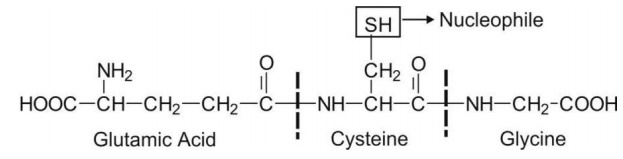
Thus, it has great affinity for electrophilic
substrates, a number of which are potentially toxic compounds. It is important
to note that a highly electrophilic metabolite has a tendency to react with
tissue nucleophilic groups such as -OH, -NH2 and -SH and precipitate
toxicities such as tissue necrosis, carcinogenesis, mutagenesis, teratogenesis,
etc. Conjugation with glutathione protects the tissue from such reactive
moieties and thus, the reaction is an important detoxication route.
GSH conjugation differs from other conjugation
reactions in that the process does not
require initial activation of the
coenzyme or the substrate since the GSH, which is a nucleophile itself, is highly reactive towards an electrophilic
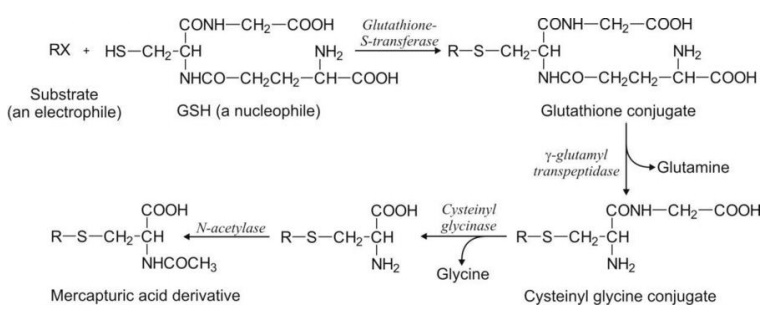
substrate. The interaction between the substrate and the GSH is catalysed by
enzyme glutathione-S-transferase to form S-substituted glutathione conjugate.
This conjugate is not excreted as such in the urine but undergoes cleavage,
first by the enzyme -glutamyl transpeptidase to release the free glutamyl
residue and cysteinyl glycine derivative; the latter is cleaved by enzyme
cysteinyl glycinase to produce free glycine and the cysteine conjugate.
Finally, N-acetylation of this conjugate by the enzyme N-acetylase yields
S-substituted-N-acetyl cysteine products called as mercapturic acids.
GSH conjugation occurs by one of the two
mechanisms:
1. Nucleophilic substitution at an
electron deficient carbon or heteroatom such as alkyl halides, alkyl nitrates (e.g. glyceryl trinitrate), sulphates,
sulphonates, organophosphates, epoxides, lactones, etc.
RX + GSH → R–S–G + H+ + X–
2. Nucleophilic
addition at the electron deficient double bond such as the unsaturated carbonyl compounds, e.g.
ethacrynic acid.
R–CH=CH–R' + GSH → RCH–S–G–CH2–R'
where R = electron withdrawing group.
The hepatotoxicity from paracetamol overdose is due
to depletion of GSH.
Related Topics
