Comments
| Home | | Pharmacovigilance |Chapter: Pharmacovigilance: NSAIDs - COX-2 Inhibitors – Risks and Benefits
The post-marketing safety assessment of COX-2 inhibitors and market withdrawal of rofecoxib provided important lessons but left some unanswered questions as of the writing of this chapter.
COMMENTS
LIMITATIONS OF SPONTANEOUS REPORTS SYSTEM
The
post-marketing safety assessment of COX-2 inhibitors and market withdrawal of
rofecoxib provided important lessons but left some unanswered questions as of
the writing of this chapter. Perhaps the most important lesson is the
limitation of spontaneous adverse drug reactions reporting system in the
detec-tion of safety signals with a high background rate in a population using
a drug of interest. In the US where millions of patients have used celecoxib or
rofecoxib in 1999 and 2000, there has been no cardiovascular safety signal
identified in the Adverse Event Report-ing System. The safety signal report
from the Nether-lands was not widely publicized in the US. If there was no
VIGOR or APPROVe, the cardiovascular risk of rofecoxib might not be recognized
until much later. It is understandable that the traditional spontaneous
reporting system did not detect the cardiovascular safety signal of rofecoxib.
For rare outcomes that have been previously reported as drug-induced adverse
events, such as Stevens–Johnson syndrome, liver fail-ure, or agranulocytosis,
the prescribing physician’s level of suspicion may be high and the adverse
event is more likely to be reported. For an adverse event like acute myocardial
infarction in which the background rate is not rare and risk factors are well
characterized, the prescribing physician may not readily attribute the
myocardial infarction in a patient to the rofecoxib that the patient was using.
For example, an overweight 59-year-old male smoker who had poorly controlled
blood pressure and serum cholesterol started rofecoxib for his knee pain and
then developed acute myocardial infarction in early 2000. Results from VIGOR
were not yet available, the event could be explained by the patient’s existing
cardiovascular risk factors (smok-ing, hypertension, and hypercholesterolemia),
and the event would not be reported as an adverse drug reaction. This example
illustrates the impor-tance of additional safety signal detection scheme to
complement the existing spontaneous reporting system.
PRE-MARKETING AND POST-MARKETING TRIALS
According
to current regulatory requirement, clini-cal trials of new NSAIDs like the
COX-2 inhibitors only need to demonstrate short-term efficacy and safety. The
study populations are usually relatively healthy and free from major
comorbidity. However, once the drug is on the market, it is used in patients
with a wide range of chronic diseases and concomi-tant medications and the new
drug is used for peri-ods much longer than the study period of the
pre-marketing trials. In all observational studies of COX-2 inhibitors that
evaluated baseline comorbidity of study subjects, a substantial proportion had
cardiovascular risk factors at baseline. Pooling data from multiple clinical
trials (Konstam et al., 2001; Weir et al., 2003; White et al., 2003; White et al.,
2004; Matchaba et al., 2005) to
increase statistical power to evaluate risk of rare events is an important tool
in safety assess-ment, but it does not address the issue of limited trial
duration and non-generalizability to patients with cardiovascular comorbidity
and concomitant medica-tions. Moreover, not all relevant safety information is
included in published reports. Zhang and colleagues identified 502 reports
involving COX-2 inhibitors and 331 had no event data on the occurrence of
arrhyth-mia or renal complications (Zhang, Ding and song, 2006).
Sample
sizes of the post-marketing trials of cele-coxib (CLASS), rofecoxib (VIGOR),
and lumira-coxib (TARGET) were much larger than that of the pre-marketing
trials and had larger statistical power to evaluate less common adverse events.
Even so, they were not powered to precisely esti-mate relative risk associated
with serious cardio-vascular outcomes. Moreover, low dose aspirin was allowed
in only two of the three trials and provided limited information on potential
interaction between COX-2 inhibitors and aspirin on gastrointestinal and
cardiovascular
outcomes. Placebo-controlled trials of COX-2 inhibitors and non-selective
NSAIDs would provide the most compelling evidence on the safety of these drugs,
but these trials are ethically infeasible. For the active-control trials,
long-term cardiovascu-lar safety of the commonly used comparator drugs, ibuprofen,
naproxen, and diclofenac, has not been evaluated in clinical trials.
Placebo-controlled results would have to come from study populations who did
not require NSAID therapy, and APPROVe, APC, and PreSAP were such studies which
demonstrated the increased cardiovascular risk among users of rofe-coxib and
celecoxib. Not surprisingly, incidence of adverse cardiovascular events was
much lower in these three trials than that observed among CLASS, VIGOR, and
TARGET, raising questions about the generalizability of these results to
patients who need NSAIDs. Trials need to be conducted among patients with
coronary heart disease or cardiovascular risk factors to provide the most valid
and generalizable answer to address the cardiovascular safety ques-tions of the
COX-2 inhibitors. The Multinational Etoricoxib and Diclofenac Arthritis
Long-Term program sponsored by the manufacturer of etori-coxib (Merck News
Release, 2006) and the Prospec-tive Randomized Evaluation of Celecoxib
Integrated Safety vs. Ibuprofen or Naproxen sponsored by the manufacturer of
celecoxib (Cleveland Clinic Press Release, 2005) will provide more definitive
answers.
Lastly,
while the analgesic and anti-inflammatory effects of the non-selective NSAIDs
may be similar at optimal doses, their cardiovascular safety profiles may not
be the same. The meta-analysis of clinical trials involving NSAIDs by Kearney
and colleagues and the meta-analysis by McGettigan and colleagues clearly
indicated that the NSAIDs are not the same with regards to adverse cardiovascular
effects (Kearney et al., 2006;
McGettigan and Henry, 2006). This heterogeneity
of cardiovascular effects has major implications in the selection of comparison
groups in large safety trials.
THE ROLE OF OBSERVATIONAL STUDIES
As
large-scale clinical trials are costly and time-consuming, evaluation of
cardiovascular safety of COX-2 inhibitors with existing automated data has been
an efficient way to provide important safety information on a timely basis.
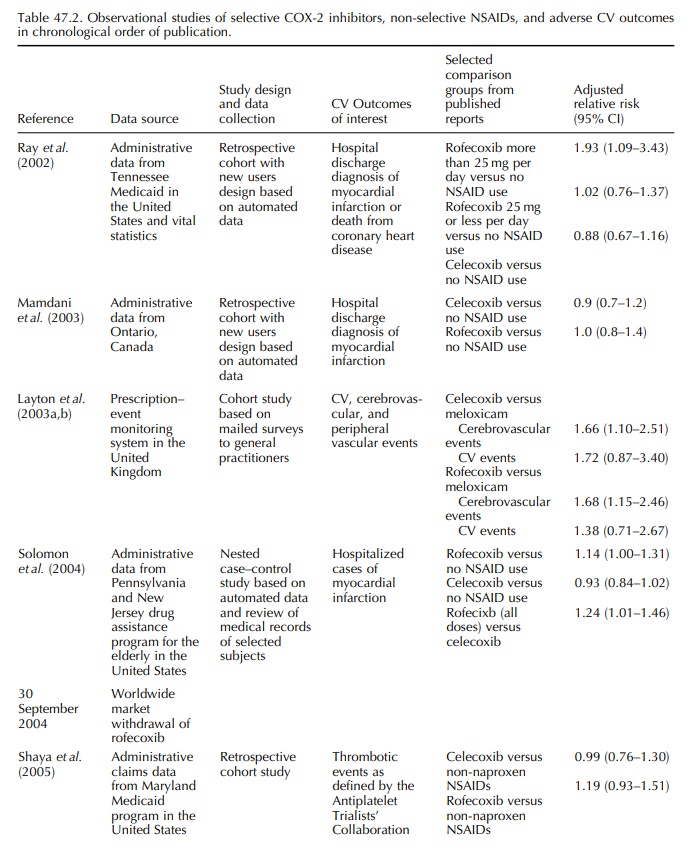
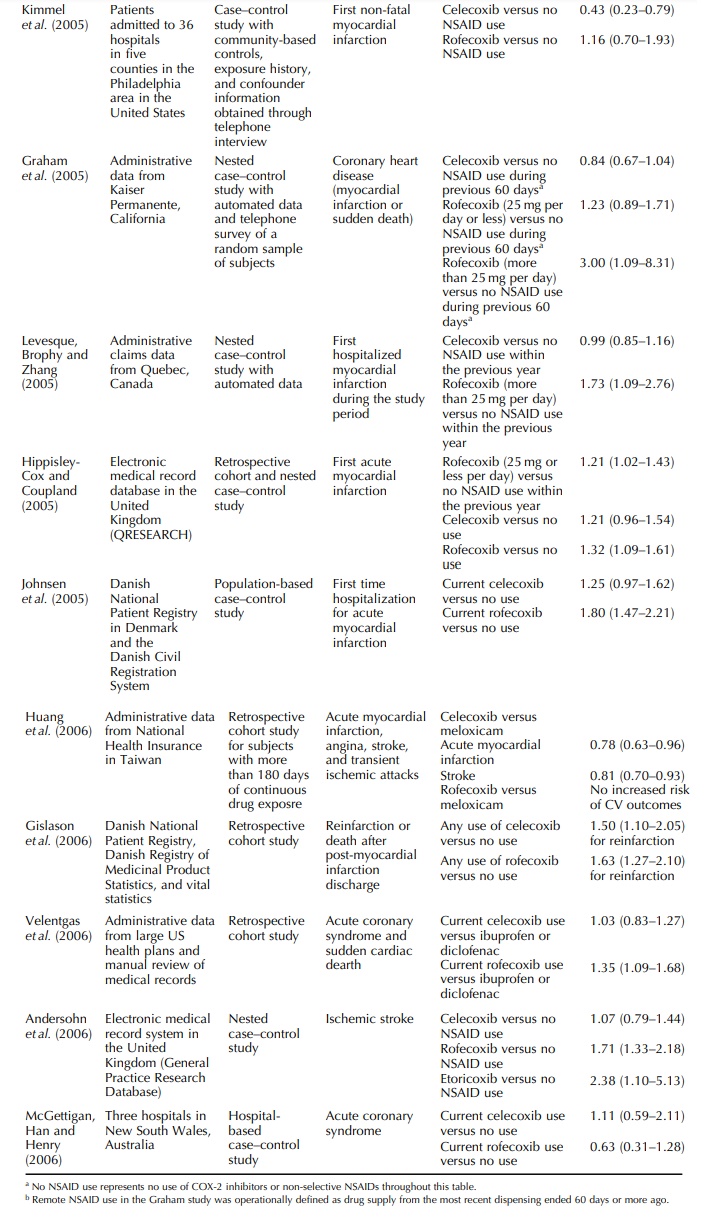
Thirteen of the fifteen studies summarized in Table 47.2 are based on
auto-mated data sources, further demonstrating the util-ity of these data
systems in rapid response to drug safety signals. The observational studies on
the COX-2 inhibitors did suggest increased risk of serious cardiovascular thrombotic
events among rofecoxib users, especially at dosages higher than 25 mg per day.
However, the study designs for these reports were not the same, the comparator
drugs were differ-ent, and important confounders, including smoking, body mass
index, and use of non-prescription low dose aspirin, were not accounted for in
the analysis in several studies.
RISK–BENEFIT ASSESSMENT OF THE COX-2 INHIBITORS AND NSAIDS
The
therapeutic role of COX-2 inhibitors needs to be interpreted in the context of
the risk–benefit profiles of the agents. Difficulties experienced by
regula-tors are discussed by a senior FDA officer (Kweder, 2004) and by the
director of the Uppsala Monitor-ing Center (Edwards, 2005). For both the COX-2
inhibitors and non-selective NSAIDs, their princi-pal anticipated beneficial
effects are the analgesic and anti-inflammatory effects, and no single agent or
class of agent has been shown to have superior efficacy than others. The risks
may involve multiple organ systems and are not restricted to the
gastroin-testinal and cardiovascular systems. Liver, renal, cutaneous, and
hematologic toxicities are impor-tant issues to consider in the risk–benefit
calculus. While the COX-2 inhibitors are associated with less gastrointestinal
complications than selected NSAIDs for patients not taking aspirin, how they
compare against the combination of NSAID and a proton pump inhibitor or an H2
blocker or misoprostol is not known.
Another
factor that may affect the risk–benefit profile of NSAIDs and COX-2 inhibitors
is the devel-opment of new indication. Celecoxib has already been shown to
decrease the development of rectal polyp among patients with familial polyposis
and it has been shown to decrease the recurrence of colorectal adenoma among
those who had a history of adenoma removal (Arber et al., 2006; Bertag-nolli et
al., 2006). The efficacy results of APPROVe will provide more information
on the potential use of COX-2 inhibitors in the prevention of colorectal
adenoma.
In
addition to overall risk–benefit assessment, regu-lators and clinicians need to
carry out the assessment among subgroups of patients defined by specific risk
factors, including those for gastrointestinal compli-cations and cardiovascular
disease. For example, the risk–benefit calculus for a 70-year-old over-weight
man who has osteoarthritis, coronary heart disease, and prior history of
gastric perforation is very different from that for a 35-year-old woman who has
no history of heart disease or gastroin-testinal complications and needs pain
medication for rheumatoid arthritis. Another issue that has not been adequately
addressed in the large COX-2 inhibitor trials is the effect of duration of
treatment, which has major clinical implications. More systematic synthe-sis of
data and quantitative risk–benefit assessment for the non-selective NSAIDs and
COX-2 inhibitors are needed.
Related Topics
