Classification of Antibiotics
| Home | | Medicinal Chemistry |Chapter: Medicinal Chemistry : Antibiotics
Antibiotics are classified on the basis of their mechanism of action and by its chemical nature.
CLASSIFICATION
Antibiotics
are classified on the basis of their mechanism of action and by its chemical
nature.
Classification Based on Mechanism of Action
1.
Agents that inhibit the synthesis of bacterial
cell wall: These include the
penicillins and cephalosporins that are structurally similar and dissimilar
agents, such as cycloserine, vancomycin, bacitracin and the imidazole
antifungal agents.
2.
Agents that act directly on the cell membrane of
the microorganisms, affecting permeability, and leading to leakage of
intracellular compounds: These
include polymyxin, polyene antifungal agents, nystatin, and amphotericin B that
bind to cell wall sterols.
3.
Agents that affect the function of 30s and 50s
ribosomal subunits to cause reversible inhibition of protein synthesis: These include tetracyclines, erythromycins,
chloramphenicol, and clindamycin.
4.
Agents that bind to the 30s ribosomal subunit
and alter protein synthesis: These
include aminoglycosides that leads to cell deaths eventually.
5.
Agents that affect nucleic acid metabolism: Such as rifamycins, which inhibit DNA dependent
RNA polymerase.
Classification Based on Chemical Structure
1.
β-lactam
antibiotics
2.
Aminoglycoside
antibiotics
3.
Tetracycline
antibiotics
4.
Polypeptide
antibiotics
5.
Macrolide
antibiotics
6.
Lincomycins
7.
Other
antibiotics
1. β-lactam
antibiotics
These
consists of two major class of agents, that is penicillins and cephalosporins.
a. Penicillins
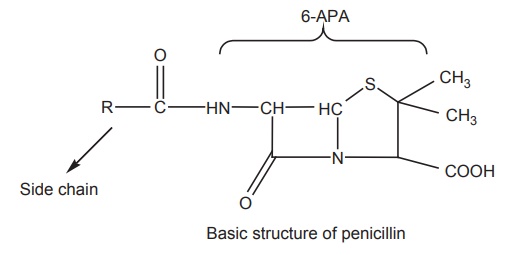
Penicillin,
the most important antibiotic, was first extracted from the mould Penicillium notatum. Subsequently, a
mutant of a related mould, P.
chrysogenum, was found to give the highest yield of penicillin and is
employed for the commercial production of this antibiotic. Penicillin belongs
to a group of antibiotics called β-lactam
antibiotics . The basic structure of the penicillins consists of a thiazolidine
ring fused with a β-lactam ring, which is essential for antibacterial activity.
These two rings constitute the fundamental nucleus of all the penicillins,
namely, 6-amino penicillanic-acid (6-APA) A variety of semisynthetic
penicillins are produced by altering the composition of the side chain attached
to 6-APA nucleus. Both the 6-APA nucleus and side chain are essential for the
antibacterial activity.
Nomenclature
Penicillins
are named in the following ways:
a. Chemical
abstract
1.
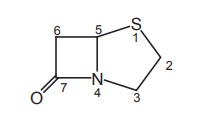
The
penicillins are described as 4-thia-1-azabicyclo (3.2.0) heptanes.
2.
Benzylpenicillin
is 6-(2-phenylacetamido)-3,
3-dimethyl-7-oxo-4-thia-1-azabiclo(3.2.0)heptane2-carboxylic acid.
b. Penam
In order to
simplify the unsubstituted bicylic ring system of penicillin, it is given the
name penam. Accordingly, the penicillins are 6-acylamino-2, 2-dimethyl
penam-3-carboxylates.
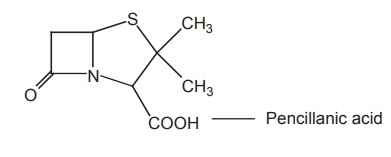
c. Pencillanic acid derivatives
Related Topics
