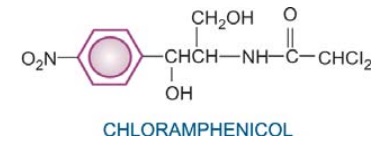
Chloramphenicol
| Home | | Pharmacology |Chapter: Essential pharmacology : Tetracyclines And Chloramphenicol (Broadspectrum Antibiotics)
Chloramphenicol was initially obtained from Streptomyces venezuelae in 1947. It was soon synthesized chemically and the commercial product now is all synthetic.
CHLORAMPHENICOL
Chloramphenicol was
initially obtained from Streptomyces
venezuelae in 1947. It was soon synthesized
chemically and the commercial product now is all synthetic.
It is a yellowish
white crystalline solid, aqueous solution is quite stable, stands boiling, but
needs protection from light. It has a nitrobenzene substitution, which is
probably responsible for the antibacterial activity and its intensely bitter taste.
Mechanism Of Action
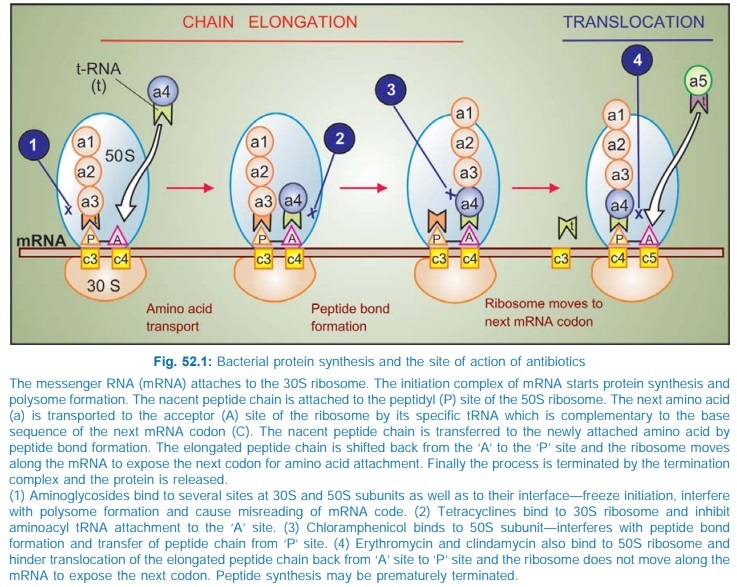
Chloramphenicol inhibits bacterial protein synthesis by
interferring with ‘transfer’ of the elongating peptide chain to the newly
attached aminoacylt-RNA at the ribosomem-RNA complex. It specifically attaches to
the 50S ribosome and thus may hinder the access of aminoacylt-RNA to the
acceptor site for amino acid incorporation (see
Fig. 52.1). Probably by acting as a peptide analogue, it prevents formation of
peptide bonds.
At high doses, it can inhibit mammalian mitochondrial protein
synthesis as well. Bone marrow cells are especially susceptible.
Antimicrobial Spectrum
Chloramphenicol is primarily bacteriostatic, though high
concentrations have been shown to exert cidal effect on some bacteria, e.g. H. influenzae. It is a broad-spectrum
antibiotic, active against nearly the same range of organisms (gram-positive
and negative bacteria, rickettsiae, mycoplasma) as tetracyclines. Notable
differences between these two are:
· Chloramphenicol was highly active against Salmonella including S. typhi, but resistant strains
are now rampant.
· It is more active than tetracyclines against H. influenzae
(though many have now developed resistance),
B. pertussis, Klebsiella, N. meningitidis
and anaerobes including Bact. fragilis.
·
It is less active against gram-positive cocci,
spirochetes, certain Enterobacteriaceae and Chlamydia.
Entamoeba and Plasmodia are not inhibited.
Like tetracyclines, it is ineffective against Mycobacteria, Pseudomonas, many Proteus, viruses and fungi.
Resistance
Most bacteria are
capable of developing resistance
to chloramphenicol, which generally emerges in a graded manner, as with
tetracyclines. Being orally active, broad-spectrum and relatively cheap,
chloramphenicol was extensively and often indiscriminately used, especially in
developing countries, resulting in high incidence of resistance among many gram-positive
and gram-negative bacteria.
In many areas, highly
chloramphenicol resistant S. typhi
have emerged due to transfer of R factor by conjugation. Resistance among gram-negative
bacteria is generally due to acquisition of R plasmid encoded for an acetyl
transferase— an enzyme which inactivates chloramphenicol. Acetyl-chloramphenicol
does not bind to the bacterial ribosome. In many cases, this plasmid has also
carried resistance to ampicillin and tetracycline. Multi-drugresistant S. typhi have arisen.
Decreased permeability
into the resistant bacterial cells (chloramphenicol appears to enter bacterial
cell both by passive as well as facilitated diffusion) and lowered affinity of
bacterial ribosome for chloramphenicol are the other mechanisms of resistance.
Partial cross resistance between chloramphenicol and erythromycin/ clindamycin
has been noted, because all these antibiotics bind to 50S ribosome at adjacent
sites. Some cross resistance with tetracyclines also occurs, though the latter
binds to 30S ribosome.
Pharmacokinetics
Chloramphenicol is
rapidly and completely absorbed after oral ingestion. It is 50–60% bound to
plasma proteins and very widely distributed: volume of distribution 1 L/kg. It
freely penetrates serous cavities and blood-brain barrier: CSF concentration is
nearly equal to that of unbound drug in plasma. It crosses placenta and is
secreted in bile and milk.
Chloramphenicol is
primarily conjugated with glucuronic acid in the liver and little is excreted
unchanged in urine. Cirrhotics and neonates, who have low conjugating ability,
require lower doses. The metabolite is excreted mainly in urine. Plasma t½ of
chloramphenicol is 3–5 hours in adults. It is increased only marginally in
renal failure: dose need not be modified.
Preparations And Administration
The commonest route of administration of chloramphenicol is
oral—as capsules; 250–500 mg 6 hourly (max. total dose 28 g), children 25–50 mg/kg/day.
Significant bioavailability differences among different market preparations
have been shown. It is also available for application to eye/ear, but topical
use at other sites is not recommended.
CHLOROMYCETIN, ENTEROMYCETIN, PARAXIN, 250 mg, 500 mg cap, 1%
eye oint, 0.5% eye drops, 5% ear drops, 1% applicaps.
Chloramphenicol Palmitate (CHLOROMYCETIN PALMITATE,
ENTEROMYCETIN, PARAXIN 125 mg/5 ml oral susp) is an insoluble
tasteless ester of chloramphenicol, which is inactive as suCh. No. It is nearly
completely hydrolysed in the intestine by pancreatic lipase and absorbed as free
chloramphenicol, but produces lower plasma concentration.
Chloramphenicol Succinate
(ENTEROMYCETIN, CHLOROMYCETIN
SUCCINATE, KEMICETINE 1 g/ vial inj, PHENIMYCIN 0.25, 0.5, 1.0 g inj. is the soluble but inactive ester which
is used in the parenteral preparations. Intramuscular injection is painful and
produces lower blood levels. It is hydrolysed in tissues to the free active
form. However, bioavailability even on i.v. injection is only 70% due to renal
excretion of the ester before hydrolysis. also VANMYCETIN 0.4%
eye drops, 250 mg opticaps, LYKACETIN 1% skin cream, 10% otic solution.
Adverse Effects
1. Bone Marrow Depression: Of all drugs, chloramphenicol
is the most important cause of aplastic anaemia, agranulocytosis, thrombocytopenia
or pancytopenia. Two forms are recognized:
a) Nondose related idiosyncratic reaction: This is rare (1 in
40,000), unpredictable, but serious, often fatal, probably has a genetic basis
and is more common after repeated courses. Aplastic anaemia is the most common
manifestation. Apparently, a longer latent period of onset of marrow aplasia is
associated with higher mortality. Many victims, even if they survive, develop
leukaemias later.
b) Dose and duration of therapy related myelosuppression: a
direct toxic effect, predictable and probably due to inhibition of
mitochondrial enzyme synthesis. This is often reversible without long-term sequelae.
Liver and kidney disease predisposes to such toxicity.
2. Hypersensitivity
Reactions: Rashes, fever, atrophic glossitis, angioedema are infrequent.
3. Irritative
Effects: Nausea, vomiting, diarrhoea,
pain on injection.
4. Superinfections: These are similar to tetracyclines, but less
common.
5. Gray Baby Syndrome: It occurred when high
doses (~100 mg/kg) were given prophylactically to neonates, especially
premature. The baby stopped feeding, vomited, became hypotonic and hypothermic,
abdomen distended, respiration became irregular; an ashen gray cyanosis developed
in many, followed by cardiovascular collapse and death. Blood lactic acid was
raised.
It occurs because of
inability of the newborn to adequately metabolize and excrete chloramphenicol.
At higher concentration, chloramphenicol blocks electron transport in the liver,
myocardium and skeletal muscle, resulting in the above symptoms. It should be
avoided in neonates, and even if given, dose should be ~ 25 mg/kg/day.
Interactions Chloramphenicol inhibits metabolism of
tolbutamide, chlorpropamide, warfarin, cyclophosphamide and phenytoin. Toxicity
can occur if dose adjustments are not done. Phenobarbitone, phenytoin, rifampin
enhance chloramphenicol metabolism → reduce its concentration → failure of therapy may
occur.
Being bacteriostatic,
chloramphenicol can antagonize the cidal action of βlactams/
aminoglycosides on certain bacteria.
Uses
Because of serious
(though rare) bone marrow toxicity:
1.
Never use chloramphenicol for minor infections
or those of undefined etiology.
2.
Do not use chloramphenicol for infections treatable
by other safer antimicrobials.
3.
Avoid repeated courses.
4. Daily dose not to exceed 2–3 g; duration of
therapy to be < 2 weeks, total dose in a course < 28 g.
5. Regular blood counts (especially reticulocyte
count) may detect dose-related bone marrow toxicity but not the idiosyncratic
type.
6. Combined formulation of chloramphenicol with
any drug meant for internal use is banned in India.
Indications Of Chloramphenicol
Are:
1. Enteric
Fever: Chloramphenicol was
the first antibiotic and the drug of choice
for typhoid fever till the 1980s when resistant S. typhi emerged and spread globally, including most parts of
India. As a result, it became clinically unreliable; 50–80% isolates showed in vitro resistance. Many of these are
multidrug resistant—not responsive to ampicillin and cotrimoxazole as well.
However, few recent reports from certain parts of India indicate return of
sensitivity to chloramphenicol. Being orally active and inexpensive, it may be
used only if the local strain is known to be sensitive. The dose is 0.5 g 6 hourly
(children 50 mg/kg/day) till fever subsides, then 0.25 g 6 hourly for another
5–7 days, because bacteriological cure takes longer.
Being bacteriostatic, relapses occur in ~ 10% chloramphenicol
treated patients. Also, it does not prevent or cure the carrier state.
Bactericidal action is required to eradicate carrier state, because in this
state, host defence mechanisms do not operate against the pathogenic bacteria;
body treats them as commensals.
2. Pyogenic
Meningitis: Third generation cephalosporins
(± vancomycin) are presently the first line drugs for empirical therapy of
bacterial meningitis (see Ch. No.
51). Chloramphenicol in a dose of 50–75 mg/kg/day may be used as a second line
drug for H. influenzae and
meningococcal meningitis, especially in young children and cephalosporin
allergic patients, because it has excellent penetration into CSF and clinical
efficacy has been demonstrated.
3. Anaerobic Infections: caused by Bact. fragilis and others (wound
infections, pelvic and brain abscesses, etc.) respond well to chloramphenicol.
However, clindamycin or metronidazole are preferred for these. Chloramphenicol
may be used in addition or as an alternative in patients not tolerating these
drugs. A penicillin/cephalosporin is generally combined since most of these are
mixed infections.
4. Intraocular
Infections: Chloramphenicol given systemically attains high concentration in
ocular fluid. It is the preferred drug for endophthalmitis caused by sensitive
organisms.
5. As Second Choice Drug
a) to tetracyclines
for brucellosis and rickettsial infections, especially in young children and
pregnant women in whom tetracyclines are contraindicated.
b) to erythromycin for
whooping cough.
6. Urinary
Tract Infections Use of chloramphenicol
is improper when safer
drugs are available. It should be used only when kidney substance is involved
and the organism is found to be sensitive only to this drug.
7. Topically In conjunctivitis, external
ear infections—chloramphenicol 0.5–5.0% is highly effective. Topical
use on skin or other areas is not recommended because of risk of sensitization.
