Chemistry, Biosynthesis and Degradation
| Home | | Pharmacology |Chapter: Essential pharmacology : Prostaglandins, Leukotrienes (Eicosanoids) and Platelet Activating Factor
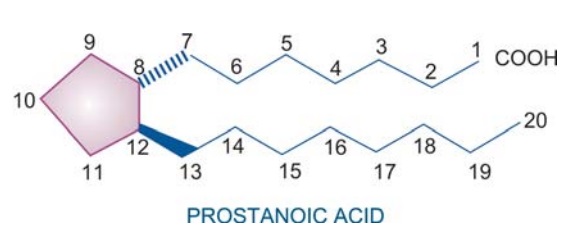
Chemically, PGs may be considered to be derivatives of prostanoic acid, though prostanoic acid does not naturally occur in the body. It has a five membered ring and two side chains projecting in opposite directions at right angle to the plane of the ring. There are many series of PGs and thromboxanes (TXs) designated A, B, C....I,
CHEMISTRY,
BIOSYNTHESIS AND DEGRADATION
Chemically, PGs may be
considered to be derivatives of prostanoic
acid, though prostanoic acid does not naturally occur in the body. It has a
five membered ring and two side chains projecting in opposite directions at
right angle to the plane of the ring. There are many series of PGs and
thromboxanes (TXs) designated A, B, C....I, depending on the ring structure and
the substituents on it. Each series has members with subscript 1, 2, 3 indicating
the number of double bonds in the side chains.
Leukotrienes are so
named because they were first obtained from leukocytes (leuko) and have 3 conjugated double bonds (triene). They have also been similarly designated A, B, C.....F and
given subscripts 1, 2, 3, 4.
In the body PGs, TXs
and LTs are all derived from eicosa (referring to 20 C atoms) tri/tetra/ penta
enoic acids. Therefore, they can be collectively called eicosanoids. In human tissues, the fatty acid released from
membrane lipids in largest quantity is 5,8,11,14
eicosa tetraenoic acid (arachidonic
acid). During PG, TX and prostacyclin
synthesis, 2 of the 4 double bonds of arachidonic acid get saturated in the
process of cyclization, leaving 2 double bonds in the side chain. Thus,
subscript 2 PGs are most important in man, e.g. PGE2, PGF2α, PGI2, TXA2.
No cyclization or reduction of double bonds occurs during LT synthesis— the LTs
of biological importance are LTB4, LTC4, LTD4.
Eicosanoids
are the most universally distributed autacoids in the body. Practically every
cell and tissue is capable of synthesizing one or more types of PGs or LTs. The
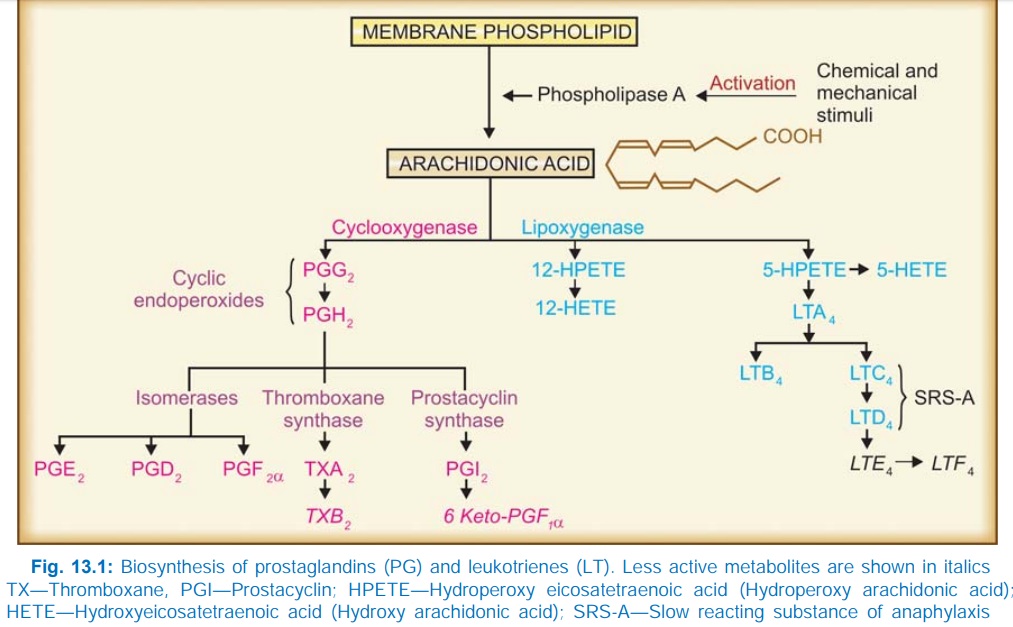
pathways of biosynthesis of eicosanoids are summarized in Fig. 13.1.
There are no preformed
stores of PGs and LTs. They are synthesized locally at rates governed by the
release of arachidonic acid from membrane lipids in response to appropriate
stimuli. These stimuli activate hydrolases, including phospholipase A, probably
through increased intracellular Ca2+.
The cyclooxygenase (COX) pathway generates
eicosanoids with a ring structure (PGs, TXs, prostacyclin) while lipoxygenase (LOX) produces open chain
compounds (LTs). All tissues have COX—can form cyclic endoperoxides PGG2
and PGH2 which are unstable compounds. Further course in a
particular tissue depends on the type of isomerases or other enzymes present in
it. PGE2 and PGF2α are the primary
prostaglandins (name based on the separation procedure: PGE partitioned into Ether while PGF into phosphate [Fosfat in Swedish] buffer; α in PGF2α refers to orientation
of OH group on the ring). PGs A, B and C are not found in the body: they are
artifacts formed during extraction procedures. Lung and spleen can synthesize
the whole range of COX products. Platelets primarily synthesize TXA2
which is —chemically unstable, spontaneously changes to TXB2.
Endothelium mainly generates prostacyclin (PGI2); also chemically
unstable and rapidly converts to 6keto PGF1α.
Cyclooxygenase
is now known to exist in two isoforms COX1 and COX2. While both isoforms
catalyse the same reactions, COX1 is a constitutive enzyme in most cells—its
activity is not changed once the cell is fully grown. On the other hand, COX2
normally present in insignificant amounts, is inducible by cytokines, growth
factors and other stimuli during the inflammatory response. It is believed that
eicosanoids produced by COX1 participate in physiological (house keeping)
functions such as secretion of mucus for protection of gastric mucosa,
haemostasis and maintenance of renal function, while those produced by COX2
lead to inflammatory and other pathological changes. However, certain sites in
kidney and brain constitutively express COX2 which may play physiological role.
A
splice variant of COX1 (designated COX3) has been found in the dog brain. This
isoenzyme is inhibited by paracetamol, but its role in humans is not known.
Lipoxygenase pathway appears to
operate mainly in the lung, WBC and
platelets. Its most important products are the LTs, (generated by 5LOX)
particularly LTB4 (potent chemotactic) and LTC4, LTD4
which together constitute the ‘slow reacting substance of anaphylaxis’ (SRSA)
described in 1938 to be released during anaphylaxis. A membrane associated
transfer protein called FLAP (five lipoxygenase activating protein) carrys
arachidonic acid to 5LOX, and is essential for the synthesis of LTs. Platelets
have only 12LOX.
HPETEs produced by LOX
can also be converted to hepoxilins, trioxilins
and lipoxins. A third enzymatic pathway
involving cytochrome P450 can metabolize arachidonic acid into 19 and 20HETEs and epoxyeicosatrienoic
acids. Free radicals can attack arachidonic acid to produce isoprostanes
nonenzymatically. Brain cells couple arachidonic acid with ethanolamine to
produce anandamide which has
cannabinoid like action. The above named
metabolites of arachidonic acid have a variety of vascular, inflammatory and
other actions, but their pathophysiological role is not clear.
Inhibition of synthesis Synthesis of COX products can be inhibited by nonsteroidal anti-inflammatory
drugs (NSAIDs). Aspirin acetylates COX at a serine residue and causes
irreversible inhibition while other NSAIDs are competitive and reversible inhibitors.
Most NSAIDs are nonselective COX1 and COX2 inhibitors, but some newer ones like
celecoxib, rofecoxib are selective for COX2.
The sensitivity of COX
in different tissues to inhibition by these drugs varies; selective inhibition
of formation of some products may be possible
at lower doses. NSAIDs
do not inhibit the production of LTs: this may even be increased since all the
arachidonic acid becomes available to the LOX pathway.
Zileuton inhibits LOX and
decreases the production of LTs. It was
used briefly in asthma, but has been withdrawn.
Glucocorticosteroids
inhibit the release of arachidonic acid from membrane lipids (by stimulating
production of proteins called annexins
or lipocortins which inhibit
phospholipase A2) — indirectly reduce production of all eicosanoids—
PGs, TXs and LTs. Moreover, they inhibit the induction of COX2 by cytokines at
the site of inflammation.
Degradation of arachidonates occurs rapidly in most tissues, but fastest in the lungs.
Most PGs, TXA2 and prostacyclin have plasma t½ of a few seconds to a
few minutes. First a specific carrier mediated uptake into cells occurs, the
side chains are then oxidized and double bonds are reduced in a stepwise manner
to yield inactive metabolites. Metabolites are excreted in urine. PGI2
is catabolized mainly in the kidney.
Related Topics
