Basic Chemistry
| Home | | Anatomy and Physiology | | Anatomy and Physiology Health Education (APHE) |Chapter: Anatomy and Physiology for Health Professionals: Levels of Organization : Chemical Basics of Life
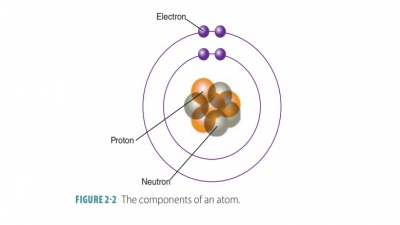
Studying basic chemistry and its relation to anatomy and physiology is important as the entire human body is made up of chemicals.
Basic
Chemistry
Studying basic chemistry and its
relation to anatomy and physiology is important as the entire human body is
made up of chemicals. Basic chemistry takes into account matter, the states of
matter, and energy in all its various types. This chapter focuses on both basic chemistry
and biochemistry.
Matter is a term that describes all things occupy-ing space and
having mass. Most types of matter can
be sensed in various forms. Mass is not exactly the same as weight. An object’s mass is equal to its
actual amount of matter. Mass remains constant regardless of where the object
is located. Weight is different because it varies with gravity. For example,
because of differ-ences in gravitational pull, your body weighs slightly more
at extremely low sea levels and slightly less at extremely high sea levels.
However, your body’s mass is exactly the same at both levels. Chemistry studies
the nature of matter and how chemical building blocks interact and how they are
constructed.
The various states of matter are gaseous,
liquid, and solid. The human body
contains examples of all three states. Gases have no shape or definite volume.
An example is the air that we breathe. Liquids
have definite volume but are “shaped” by the structure containing them. An
example is blood plasma, which assumes the shape of the blood vessels. Solids have a definite shape and volume.
Examples include teeth and bones.