Barium: rat poison or radio-contrast agent?
| Home | | Inorganic Pharmaceutical Chemistry |Chapter: Essentials of Inorganic Chemistry : Alkaline Earth Metals
The element barium (Ba) has the atomic number 56 and is classified as a heavy metal. Barium metal is highly reactive and therefore no elemental barium exists in nature.
Barium:
rat poison or radio-contrast agent?
The element barium (Ba) has the atomic number 56 and is
classified as a heavy metal. Barium metal is highly reactive and therefore no
elemental barium exists in nature. Natural sources of barium are the
water-insoluble minerals barite (barium sulfate) and whiterite (barium
carbonate). In order to obtain pure barium compounds, the mineral barite is
reacted with carbon, and barium sulfide is formed. Barium sulfide is, in
contrast to barium sulfate, water soluble. Subsequently, the pure barium
sulfide is treated with sulfuric acid and pure barium sulfate can be obtained.
BaSO4 + 4C → BaS + 4CO
BaS + H2SO4 → BaSO4 + H2S
Barium salts can be highly toxic even at low concentrations.
Barium carbonate is highly toxic and can be used as rat poison as it readily
dissolves in the stomach acid. Barium sulfate is the least toxic barium
compound mainly because of its insolubility. Barium sulfate is used in a
variety of applications ranging from white paint to X-ray contrast agent.
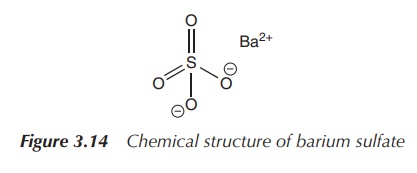
The clinical use of barium sulfate suspension is well known
under the term barium meal. Patients
are given a suspension of barium sulfate to swallow. Using X-ray imaging, the
whole oesophagus, the stomach and the intestines can be visualised. Barium
sulfate lines the tissue whilst travelling through the digestive tract. The
heavy barium ions absorb X-rays readily and therefore these structures become
visible in an X-ray screening. Barium sulfate is a well-used and tolerated oral
radio-contrast agent. It is also used as radio-contrast agent in enemas (Figure
3.14) .
Related Topics
