Applications of Microorganisms in the Partial Synthesis of Pharmaceuticals
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : The Wider Contribution Of Microbiology To The Pharmaceutical Sciences
Microorganisms and microbially derived enzymes continue to play a significant role in the production of novel antibiotics. The potential of microorganisms as chemical catalysts, however, was a later development and first realized in the synthesis of industrially important steroids.
APPLICATIONS OF MICROORGANISMS IN THE PARTIAL SYNTHESIS OF
PHARMACEUTICALS
Microorganisms and microbially derived enzymes continue to play a
significant role in the production of novel antibiotics. The potential of
microorganisms as chemical catalysts, however, was a later development and
first realized in the synthesis of industrially important steroids. These
reactions assumed increasing importance following the discovery that certain
steroids could be formulated as potent therapeutics, e.g. hydrocortisone has
anti-inflammatory activity, and derivatives of the steroidal sex hormones are
useful as oral contraceptive agents. More recently, chiral inversion of non-steroidal
anti-inflammatory drugs (NSAIDs) has also been demonstrated.
A) Production Of Antibiotics
In the antibiotics industry, the hydrolysis of benzylpenicillin to give
6-aminopenicillanic acid by the enzyme penicillin acylase is an important stage
in the synthesis of many clinically useful penicillins. The combination of
genetic engineering techniques to produce hybrid microorganisms with
significantly higher acylase levels, together with their entrapment in gel
matrices, which appears to improve the stability of the hybrids, has resulted
in considerable increases in 6-aminopenicillanic acid yields.
A second example is provided by the production by fermentation of
cephalosporin C, which is used solely for the subsequent preparation of semisynthetic
cephalosporins.
Furthermore, antibiotics produced by
fermentation of various moulds and particularly Streptomyces spp.,
can be utilized by medicinal chemists as starting blocks in the production of
what might be more effective antimicrobial compounds.
B) Steroid Biotransformations
Previously, steroid hormones could only
be obtained in small quantities directly from mammals and therefore attempts
were made to synthesize them from plant sterols, which can be obtained
economically in large quantities. However, adrenocortical steroids are
characterized by the presence of an oxygen molecule at position 11 in the steroid
nucleus and although it is relatively easy to hydroxylate a steroidal compound
it is extremely difficult to achieve site-specific hydroxylation, such that
many of the routes used for synthesizing the desired steroid are lengthy,
complex and consequently expensive. This problem was overcome when it was
realized that many microorganisms are capable of performing limited oxidations
with both stereo-and regio-specificity. By simply adding a steroid to growing
cultures of the appropriate microorganism, specific site-directed chemical
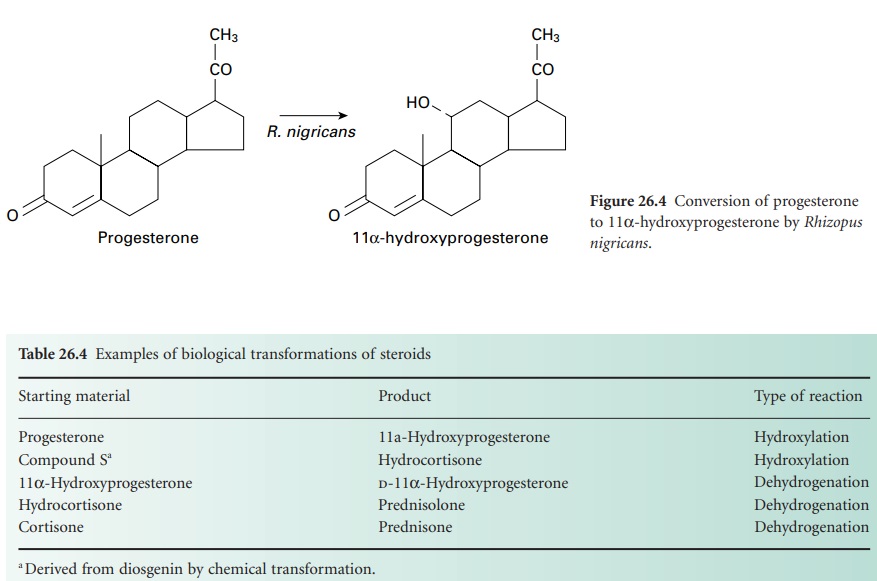
changes can be introduced into the molecule. In 1952, the first commercially
employed process involving the conversion of progesterone to 11
α-hydroxyprogesterone by the fungus Rhizopus nigricans was
introduced (Figure 26.4).
This reaction is an important stage in the manufacture of cortisone and
hydrocortisone from more readily available steroids. Table 26.4 gives
several other examples of microbially directed oxidations that have been or are
employed in the manufacture of steroidal drugs.
More recently, microorganisms utilized for biotransformation reactions
have been immobilized by entrapment in a polymer gel matrix to avoid the often
costly and time-consuming enzyme extraction steps that can result in enzyme
inactivation. Immobilization also serves to increase the stability of membrane-associated
enzymes that are unstable in the solubilized state, as well as permitting the
conversion of water-insoluble compounds like steroids in two-phase
water–organic solvent systems.
C) Chiral Inversion
Several clinically relevant drugs
including salbutamol (a β-adrenoceptor agonist), propanolol (a β-adrenoceptor
antagonist) and the 2-arylpropionic acids (NSAIDs) are administered in their
racemic form but undergo in vivo chiral
inversion through metabolic transformations by microorganisms that mimic phase
I metabolic processes, i.e. functionalization reactions. For example, the
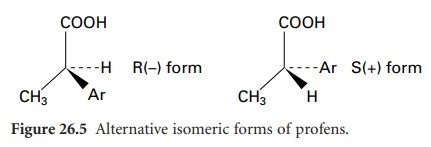
activity of NSAIDs (e.g. ibuprofen), resides almost exclusively in the S(+) isomers. However, unidirectional chiral inversion
from R (–) to S(+) (Figure 26.5)
occurs in vivo over a 3-hour period. The S(+) form is a more effective inhibitor of prostaglandin
synthesis, and enzymes from some fungal species (e.g. Verticillium lecanii) convert a racemic mixture into
the S(+) isomer in vitro. Another
example is the biotransformation of ±propranolol to S(+) propranolol by R. arrhizus and Geotrichum candidum with around 83% efficiency.
Related Topics
