Anticholinesterases - Mechanism of Action
| Home | | Pharmacology |Chapter: Essential pharmacology : Cholinergic System And Drugs
The antiChEs react with the enzyme essentially in the same way as ACh. The carbamates and phosphates respectively carbamylate and phosphorylate the esteratic site of the enzyme.
MECHANISM OF ACTION
The antiChEs react
with the enzyme essentially in the same way as ACh. The carbamates and
phosphates respectively carbamylate and phosphorylate the esteratic site of the
enzyme.
The mammalian AChE has
been cloned and details of its structure as well as mode of interaction with
ACh and various antiChEs has been worked out.
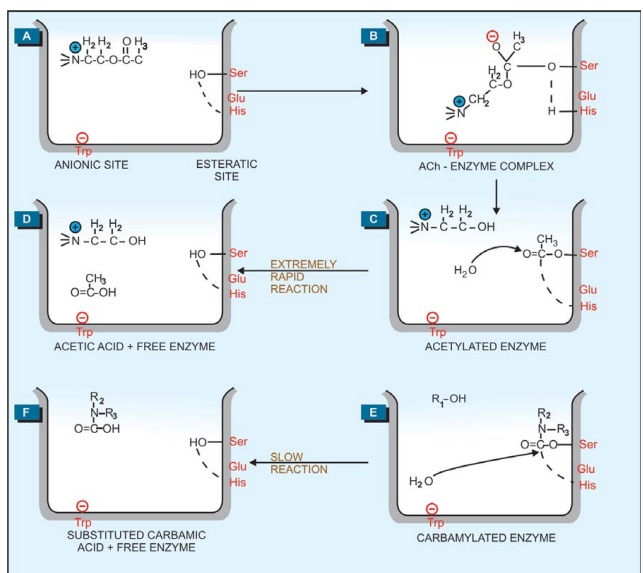
The active region of
AChE forms a gorge which contains an aromatic anionic site (near tryptophan 86) and an esteratic site formed by
serine 203, glutamate 334, histidine 447 (Fig.
7.2A). Hydrolysis of ACh involves electrostatic attraction of positively
charged N+ of ACh to the aromatic pocket (Fig. 7.2B) and
nucleophilic attack by serineOH which is activated by the adjacent histidine
leading to acetylation of serine (Fig. 7.2C). The acetylated enzyme reacts with
water to produce acetic acid and choline (Fig. 7.2D).
Whereas the acetylated
enzyme reacts with water extremely rapidly and the esteratic site is freed in a
fraction of a millisecond, the carbamylated enzyme (reversible inhibitors)
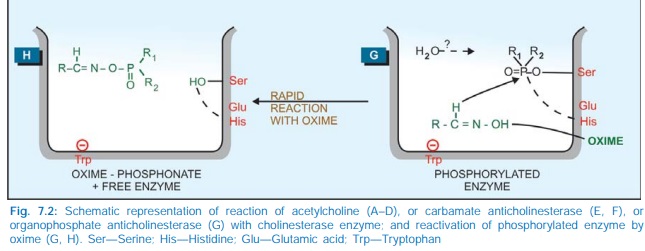
reacts slowly (Fig. 7.2E, F) and the phosphorylated enzyme (irreversible
inhibitors) reacts extremely slowly or not at all (Fig. 7.2G). It is noteworthy
that edrophonium and tacrine attach only to the anionic site of the enzyme,
while organophosphates attach only to the esteratic site. Reactivation of
edrophonium and tacrine inhibited enzyme does not involve hydrolysis of the inhibitor,
but only its diffusion—action is brief. The halflife of reactivation of
carbamylated enzyme (about 30 min) is less than that of synthesis of fresh
enzyme protein, while that of phosphorylated enzyme is more than the
regeneration time. The phosphorylated enzyme may also undergo ‘aging’ by the
loss of one of the alkyl groups and become totally resistant to hydrolysis.
Thus, apparently reversible and irreversible enzyme inhibition is obtained,
though the basic pattern of inhibitorenzyme interaction remains the same.
Related Topics
