Antibonding Orbitals
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Functional Groups and Chemical Bonding
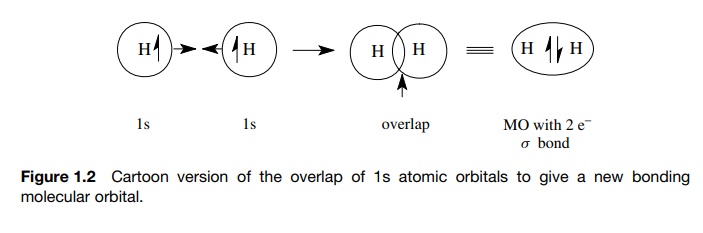
The overlap of AOs to give a new MO in which an electron pair is shared by the interacting atoms was illustrated in Figure. The new MO, which contains the shared electron pair, is of lower energy than the AOs from which it was produced by overlap.
ANTIBONDING ORBITALS
The
overlap of AOs to give a new MO in which an electron pair is shared by the
interacting atoms was illustrated in Figure 1.2. The new MO, which contains the
shared electron pair, is of lower energy than the AOs from which it was
produced by overlap. This energy change (ΔE) is illustrated in Figure 1.3 (N
represents the nucleus of some
element in the bond formation process). The ΔE is related closely to the bond
energy of the bond produced. The same model holds irrespective of the type of
AOs which overlap (simple AOs or hybrid AOs) or the type of bond formed (σ or π
).
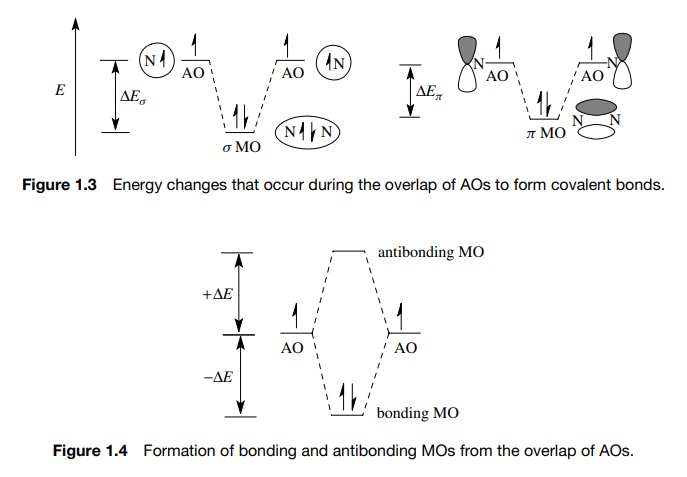
While this model is easy to visualize and understand, it is actually only half of the story. When AOs interact, the number of new MOs which are produced from that interaction must equal the number of AOs which initially interact.
Furthermore, for each MO produced which is lower in energy than the energy of
the interacting AOs, there will be one produced which will be higher in energy by the same amount
(Figure 1.4). So when two half-filled AOs interact, there will be two MOs
produced, one of lower energy which will contain the electron pair and is
termed the bonding MO. The second
molecular orbital is of higher energy, is unfilled, and is termed the antibonding MO.
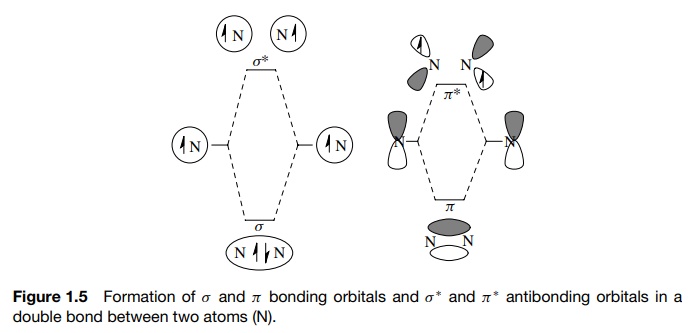
For
each bond in a molecule which is described by the overlap of AOs, there will be
a bonding MO which is of lower energy and when filled with an electron pair
gives rise to a stable bond between elements. There will also be an antibonding
MO which is of higher energy and thus unfilled. Antibonding orbitals correspond
to the situation where nuclei are moved to within the bonding distance of one
another but there is no electron
sharing; in fact the electrons and nuclei actually repel one another. This
electronic and nuclear repulsion is what increases the energy of the
antibonding level. Because the bonding MO is filled and the antibonding MO is
unfilled, the system is at a lower net energy than the individual AOs and bond
formation takes place. This occurs for both σ
and π bonds as shown in Figure 1.5 (the antibonding orbitals are indicated by
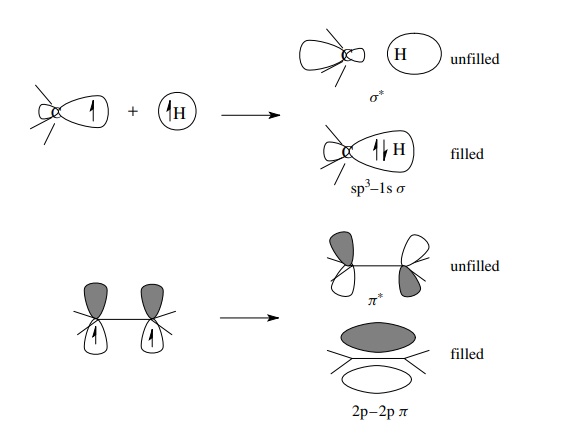
the asterisk). Overlap of an sp3 AO on a carbon with a 1s AO on a
hydrogen gives a σ -bonding MO that
is filled with two electrons and an unfilled, higher energy, antibonding MO termed
a σ ∗ MO. Likewise, overlap of two 2p AOs on carbon gives a π MO which contains a shared pair of e− and a π ∗ MO which is of
higher energy and is unfilled.
Thus
far it would appear that antibonding orbitals are real orbitals, but they seem
to be merely mathematical artifacts since they are unfilled and thus do not
enter into bonding or energy considerations. For ground-state molecules this is
actually true — all of the electrons are found in bonding orbitals. Why, then,
should we even concern ourselves with their existence?
The
answer lies in the realization that antibonding orbitals are still, in fact,
orbitals. They are regions of space where one could have electrons. In
ground-state molecules, electrons fill the lower energy bonding orbitals.
Suppose, how-ever, you wished to take an electron out of a bonding orbital and
move it to a higher level. Where would it go? Or suppose you wished to add
electrons to a molecule which already had its bonding orbitals filled. Where
would the electrons go? Suppose an electron-rich reagent were to donate
electrons to a molecule. Where would the electrons go?
In
these examples the electrons could go into a higher energy, unfilled MO which
could be a nonbonding orbital (when one is present) or an antibonding orbital
(which is always present). Thus it is most common to have the elec-trons go
into an antibonding MO. Although they are of high energy, antibonding orbitals
are usually unfilled and can accept electrons from several sources if sufficient
energy is available to promote electrons into the antibonding energy level.
Absorption of light energy can cause an electron to be promoted from the
highest occupied molecular orbital (HOMO), which is usually a bonding MO, to
the lowest unoccupied molecular orbital (LUMO), which is most often an
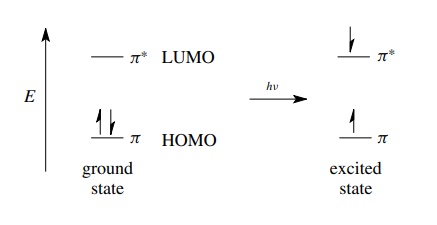
antibonding MO. For example, if an olefin which contains a carbon – carbon π bond is exposed to ultraviolet light
of the correct frequency (and hence energy), the molecule can absorb the energy
of the light by promoting a π
electron from the bonding MO into the antibonding MO. This new electronic state
is termed an excited state and is higher in energy than the initial
electron-paired state called the ground state. (The electron spins can be
paired in the singlet excited state or unpaired in the triplet excited state.)
Excited states of molecules are high-energy states which are much more reactive
than ground states and can be described in terms of the population of
antibonding orbitals. Consequently, almost all photochemical reactions which
occur by the reactions of excited-state species are intimately dependent on the
existence of and population of antibond-ing orbitals.
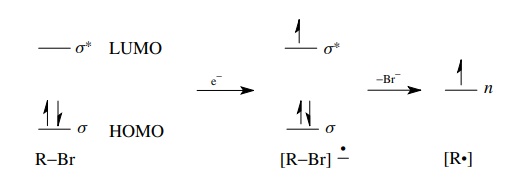
The
reduction of organic molecules by the addition of electrons can take place by
chemical reagents or at the surface of electrodes. In either case electrons are
added to the organic compound, thus reducing it. Now electrons cannot just go
anywhere; they must go into an unfilled orbital. Thus, during a reduction, electrons
are injected into the LUMO of the molecule, which is often an anti-bonding
orbital. Population of the antibonding orbital raises the total energy of the
molecule and subsequent reactions follow. The electrochemical reduction of
alkyl bromides illustrates the process well. An electron is added into the σ ∗ orbital of the
carbon – bromine bond, which is the LUMO of a saturated alkyl bromide.
Population of the antibonding orbital raises the energy of the molecule and
weakens the carbon – bromine bond, which then dissociates to give bromide ion
and a carbon-centered free radical which has an unpaired electron in a hybrid
AO (nonbonded energy level).
Almost
all dissolving metal and electrochemical reductions follow this same general
sequence. An electron is donated into an unfilled orbital which is usu-ally an
antibonding MO, the energy of the molecule is raised, and chemical change
ensues.
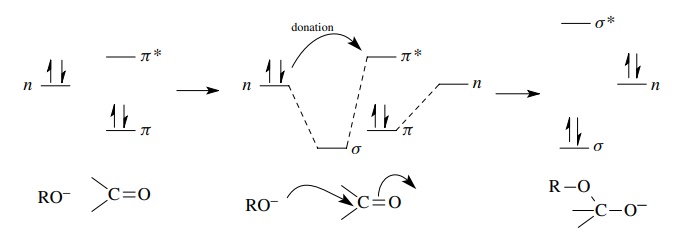
When
a nucleophile attacks an electrophile, it donates a pair of electrons to the
electrophile. Electron donation must take place by an overlap interaction
between a filled orbital on the nucleophile which contains the electron pair to
be donated and an unfilled orbital (LUMO) on the electrophile, which is usually
an antibonding orbital. Population of the LUMO by electron donation raises the
energy of the system leading to bonding change and new bond formation. Addition
of an alkoxide to a ketone is a typical example of the process. The electron
pair to be donated is in a hybrid AO and therefore is at a nonbonding energy
level (n). Overlap with the π ∗ orbital of the
carbonyl group starts to populate the π
∗ orbital. This weakens the π bond, and the carbon – oxygen π bond of the carbonyl group is broken
and a new lower energy σ bond is
formed between the oxygen of the alkoxide and the carbonyl carbon. The
electrons of the π bond end up in a
nonbonding AO on oxygen in the product. This process is shown schematically.
Nucleophilic
additions and substitutions are the most widespread of all organic reactions,
and all have the same general orbital requirements. An orbital containing an
electron pair of the nucleophile overlaps with an antibonding orbital of the
electrophile, which leads to population of the antibonding level (in most
cases). This raises the energy of the system and bond and electron
reorganization follows to give products. The electron pair must be able to be
donated (i.e., not tightly bound or of higher energy) and the antibonding
orbital be of sufficiently low energy to ensure effective overlap.
Thus
it is seen that, although antibonding orbitals are not a major factor in
describing the bonding of ground-state molecules, they can play a pivotal role
in the reactions of molecules. Therefore it is important to keep in mind the
existence of antibonding orbitals and their ability to accept electrons and
control the reactivity of molecules.
