Anti-Herpes Virus Drugs
| Home | | Pharmacology |Chapter: Essential pharmacology : Antiviral Drugs
Idoxuridine It is 5iodo2deoxyuridine (IUDR); acts as a thymidine analogue. It was the first pyrimidine antimetabolite to be used as antiviral drug. It competes with thymidine, gets incorporated in DNA so that faulty DNA is formed which breaks down easily
ANTIHERPES VIRUS DRUGS
Idoxuridine It is 5iodo2deoxyuridine (IUDR); acts as a thymidine analogue. It was the first
pyrimidine antimetabolite to be used as antiviral drug. It competes with
thymidine, gets incorporated in DNA so that faulty DNA is formed which breaks
down easily. It is effective only against DNA viruses and clinical utility is
limited to topical treatment of Herpes
simplex Keratitis, labial and genital herpes. However, because of low virus
selectivity, higher local toxicity and rapid development of viral resistance,
it has been superseeded by acyclovir.
Trifluridine and vidarabine are other pyrimidine antimetabolites effective against H. simplex.
Acyclovir
This deoxiguanosine
analogue antiviral drug requires a virus specific enzyme for conversion to the active metabolite that inhibits DNA
synthesis and viral replication.
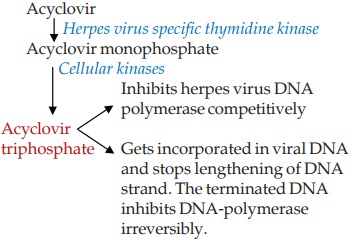
Acyclovir is preferentially taken up by the virus infected
cells. Because of selective generation of the active inhibitor in the virus
infected cell and its greater inhibitory effect on viral DNA synthesis,
acyclovir has low toxicity for host cells: a several hundredfold
chemotherapeutic index has been noted.
Acyclovir is active
only against herpes group of viruses; H.
simplex type I is most sensitive followed by H. simplex type II > varicellazoster virus=EpsteinBarr virus; while
cytomegalovirus (CMV) is practically not affected. Both H. simplex and varicellazoster
virus have been found to develop resistance to acyclovir during therapy; the former
primarily due to mutants deficient in thymidine kinase activity and the latter primarily
by change in specificity of virus directed enzyme so that its affinity for
acyclovir is decreased.
Pharmacokinetics
Only about 20% of an
oral dose of acyclovir is
absorbed. It is little plasma protein bound and is widely distributed attaining
CSF concentration that is 50% of plasma concentration. It penetrates cornea
well. Acyclovir is primarily excreted unchanged in urine, both by glomerular filtration
and tubular secretion; plasma t½ is 2–3 hours. Renal impairment necessitates
dose reduction.
ZOVIRAX 200 mg tab, 250 mg/vial for i.v. inj; CYCLOVIR 200 mg
tab, 5% skin cream; HERPEX 200 mg tab, 3% eye oint, 5% skin cream; OCUVIR 200,
400, 800 mg tab, 3% eye oint, ACIVIRDT 200, 400, 800 mg tab. ACIVIR EYE 3%
oint.
Use
Acyclovir is effective
in patients with normal as well as
deficient immune status.
1. Genital Herpes simplex: Generally caused by
type II virus; can be treated by topical, oral or parenteral acyclovir
depending on stage and severity of disease.
Primary disease: 5% ointment is applied
locally 6 times a day for 10 days. This is effective only if started early and in
mild cases. Late and more severe cases should receive oral therapy (1 g/day in
5 divided doses or 400 mg TDS for 10 days) in addition to local therapy. Both
local and oral therapies afford symptomatic relief and rapid healing of
lesions, but do not prevent recurrences.
Recurrent disease: Topical therapy is
totally ineffective. Response to oral treatment is slow and incomplete; severe
cases may be treated parenterally—5 mg/kg i.v. infused over 1 hr, repeated 8
hourly for 10 days. Suppressive oral therapy with 400 mg BD has been shown to
prevent recurrences as long as given. It is recommended to stop treatment after
1 yr and ascertain whether the patient is still having recurrences; if so restart
treatment. After prolonged therapy frequency of recurrences is reduced.
Continuous acyclovir prophylaxis is generally advocated in patients with > 8
recurrences per year. However, suppressive therapy reduces, but does not toally
prevent, disease transmission to sexual partner.
2.
Mucocutaneous H. simplex is a type I virus disease, remains localized to lips and
gums; does not usually require specific treatment, but acyclovir skin cream may
provide some relief. Spreading lesions may be treated with 10 day oral
acyclovir. Prophylactic oral therapy may prevent sun exposure related
recurrences. The disease often gets disseminated in immunocompromised individuals
and may be treated with oral or i.v. acyclovir (15 mg/kg/day) for 7 days, but
recurrences are not prevented.
3.
H. simplex encephalitis (type I virus):
Acyclovir 10 to 20 mg/kg/8 hr i.v. for >10 days is the drug of
choice. Treatment is effective only if started early: delay precludes salutary
effect on mortality and neurological complications.
4. H. simplex (type I)
keratitis: Acyclovir is equally effective as idoxuridine in superficial dendritic corneal ulcer,
and may be better for deep stromal infections because of good corneal
penetration. Though acyclovir eye ointment acts slower than idoxuridine eye drops,
blindness can be prevented. The eye ointment should be applied 5 times daily
till 3 days after healing.
5. Herpes
zoster: The varicella-zoster virus is less susceptible to acyclovir. As such, higher doses are needed and it
should be used only in immunodeficient individuals or in severe cases: 10 mg/
kg/8 hr i.v. for 7 days. Oral therapy with 800 mg 5 times daily is beneficial
only if started early. It affords symptomatic relief and faster healing of
lesions. Postherpetic neuralgia is not prevented, though its duration may be
shortened. Acyclovir skin cream may be applied on herpetic ulcers.
6. Chickenpox: in patients with
immunodeficiency and in neonates only calls for specific therapy. Acyclovir (15
mg/kg/day i.v. × 7 days) is the drug of choice: reduces fever, eruptions,
hastens healing and prevents visceral complications.
Oral acyclovir 400 mg 4 times a day for 7 days given during the
incubation period may abort chickenpox in susceptible contacts.
Adverse Effects
Topical:
stinging and burning sensation after each application.
Oral:
The drug is well tolerated; headache, nausea, malaise and some CNS effects are
reported.
Intravenous: rashes, sweating, emesis and fall in BP occur only in few patients.
Dose-dependent decrease in g.f.r. is the most important toxicity;
occurs especially in those with kidney disease; normalises on discontinuation
of the drug.
Reversible
neurological manifestations (tremors, lethargy, disorientation, hallucinations,
convulsions and coma) have been ascribed to higher doses.
No teratogenic
potential has been noted.
Valaciclovir It is an ester prodrug of acyclovir with improved oral bioavailability (55–70%)
due to active transport by peptide transporters in the intestine. During
passage through intestine and liver, it is completely converted to acyclovir in
the first passage by esterases. Thus, higher plasma levels of acyclovir are
obtained improving clinical efficacy in certain conditions; e.g. it is the drug
of choice in herpes zoster. Valaciclovir is excreted in urine as acyclovir with
a t½ of 3 hours.
Dose: For genital herpes simplex—first
episode 0.5–1.0 g BD × 10 days;
recurrent episode 0.5 g BD × 3 days; suppressive treatment 0.5 g OD × 6–12
months.
For orolabial herpes 2
g BD × 1 day; in immunocompromised patient 1 g BD × 5 days. For herpes zoster 1
g TDS × 7 days.
VALVIR 0.5 g, 1.0 g
tabs.
Famciclovir It is an ester prodrug of a guanine nucleoside analogue penciclovir, which has good oral bioavailability and prolonged
intracellular t½ of the active triphosphate metabolite. Like acyclovir, it
needs viral thymidine kinase for generation of the active DNA polymerase
inhibitor. Famciclovir inhibits H.
simplex, H. zoster but not acyclovir-resistant strains. Some activity
against hepatitis B virus (HBV) has been noted. It is used as an alternative to
acyclovir for genital or orolabial herpes and herpes zoster. Early treatment of
herpes zoster reduces the duration of post herpetic neuralgia, but not its
incidence.
Dose: Genital herpes (1st
episode) 250 mg TDS × 5 days; recurrent
cases 250 mg BD for up to 1 year. Herpes zoster and orolabial herpes 500 mg TDS
for 7–10 days.
FAMTREX 250, 500 mg
tabs.
Famciclovir is a less
active alternative to lamivudine in chronic hepatitis B, but not in resistant
cases. Side effects are headache, nausea, loose motions, itching, rashes and
mental confusion.
Ganciclovir It is an analogue of acyclovir which is active against all herpes viruses including H. simplex, H. zoster,
EB virus and
cytomegalovirus (CMV). It is more active than acyclovir against CMV. The active
triphosphate metabolite of ganciclovir attains much higher concentrations
inside CMV infected cells. The plasma t½ of ganciclovir is 2–4 hrs, but that of
its triphosphate inside CMV infected cells is > 24 hrs. These factors account
for its high activity against CMV infections. CMV can develop ganciclovir
resistance by mutation.
Systemic toxicity of ganciclovir is high (bone marrow
depression, rash, fever, vomiting, neuropsychiatric disturbances) and use is
restricted to severe CMV infections (pneumonia/colitis) in immunocompromised
(AIDS, transplant recipient) patients. Intravenous infusion of 10 mg/kg/day has
prevented blindness in AIDS patients with CMV retinitis. Ganciclovir therapy
has been found to lower HBV titre in chronic hepatitis B.
Foscarnet It is a simple straight chain phosphonate unrelated to any nucleic acid precursor which
inhibits viral DNA polymerase and reverse transcriptase. It is active against H. simplex (including strains resistant
to acyclovir), CMV (including ganciclovir resistant ones) and HIV. Viral
resistance to foscarnet is minimal. However, viral selectivity of foscarnet is
low. Oral absorption is poor. Its t½ is 4–8 hours, and it is not metabolised.
Toxicity of foscarnet is high: damages kidney— produces a renal
diabetes like condition, acute renal failure can also occur. Anaemia,
phlebitis, tremor, convulsions and other neurological as well as constitutional
symptoms due to hypocalcaemia are frequent. Administered by i.v. infusion,
foscarnet has been used for:
1. CMV retinitis and other CMV infections in AIDS patients;
efficacy is similar to ganciclovir, but includes resistant cases.
2. Acyclovir-resistant mucocutaneous H.
simplex type and varicella-zoster
infections in AIDS patients. When used to treat associated CMV/H. simplex/
Varicella-zoster infection in AIDS patient, it decreases HIV
viral titre, but is not used primarily for HIV infections.
