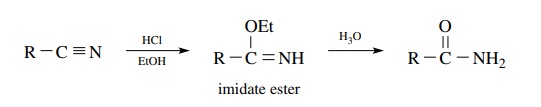Amides
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Functional Group Synthesis
Amides are usually obtained from carboxylic acids or their derivatives. The tradi-tional method of preparation of amides is to react the corresponding acid chloride with an amine.
AMIDES
Amides
are usually obtained from carboxylic acids or their derivatives. The
tradi-tional method of preparation of amides is to react the corresponding acid
chloride with an amine.
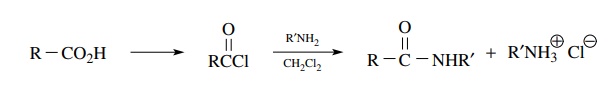
This
substitution process replaces the chloride with an amine without a change in
the oxidation level. This remains an excellent and efficient method. However,
excess amine or another base is required to neutralize the equivalent of HCl
produced by the substitution.
In
this approach, formation of an acid chloride is required to activate the car-bonyl
group and make it more electrophilic. This activation is required in most
transformations of carboxylic acids because carboxylic acids themselves are not
sufficiently electrophilic to react with most nucleophiles. Furthermore, since
many nucleophiles are also basic, they can react with carboxylic acids to give
the car-boxylate ion, which is an even poorer electrophile than the carboxylic
acid itself.
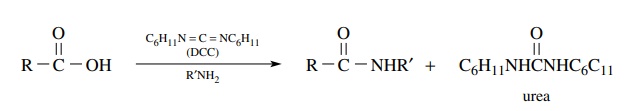
Carbodiimides
are increasingly being used to promote the conversion of car-boxylic acids to
amides. This method was originally developed for creating the amide bonds in
peptides. In this method the carboxylic acid is treated with a carbodiimide [a
very common one is dicyclohexylcarbodiimide (DCC), although many others have
been developed]. An activated acylating agent is produced which reacts with the
amine present in the mixture to produce an amide. The advantage of this
approach is that the acid is activated to a reactive electrophile in situ so
the activated species need not be isolated. Yields are normally high and the
urea by-product from the carbodiimide can be separated and removed from the
amide by one of several methods.
Amides
are also available from nitriles, which have the same oxidation level. Direct
acid or base hydrolysis of a nitrile usually requires fairly severe conditions
and often does not stop at the amide stage but goes on the carboxylic acid.
Treatment of nitriles with a solution of HCl in ethanol furnishes an imidate
ester which is hydrolyzed in aqueous acid to the amide. Because a nitrile is
the starting material, only primary amides can be produced by this process.