Alkanes
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Functional Group Synthesis
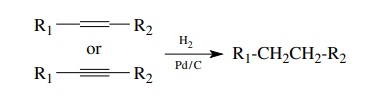
Alkanes are the most highly reduced of all organic compounds. As a consequence, virtually all preparations of alkanes are reductive. Alkenes and alkynes can both be reduced to alkanes by catalytic hydrogenation.
ALKANES
Alkanes
are the most highly reduced of all organic compounds. As a consequence,
virtually all preparations of alkanes are reductive. Alkenes and alkynes can
both be reduced to alkanes by catalytic hydrogenation. While many catalysts can
be employed, palladium on carbon is by far the most common.
Primary
and secondary alcohols can be converted to alkanes by conversion to tosylates
followed by reduction with LAH. This reduction is valuable because deuterium
can be easily introduced into the alkane by the use of lithium aluminum
deuteride (LAD) instead of LAH.
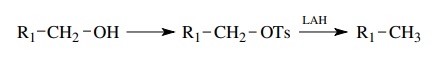
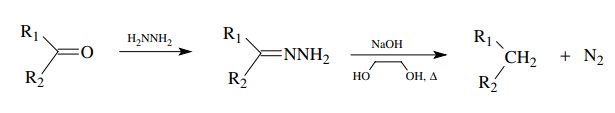
Ketones
can be reduced directly to alkanes by the Wolff – Kishner reduction. In this
reduction, the ketone is converted to the hydrazone, which is treated in situ
with sodium hydroxide. An internal redox reaction occurs in which the carbon is
reduced and the hydrazine is oxidized to nitrogen. The best experimental
conditions include the use of NaOH and ethylene glycol as solvent to carry out
the reduction.
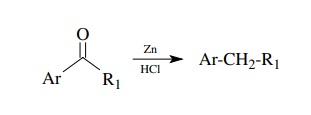
The
reduction of ketones to alkanes can also be done by the Clemmensen reduction
using zinc and HCl. This reaction is specific for aromatic ketones, however.
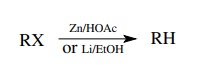
Alkyl
halides (Cl, Br, I) can be converted to alkanes by two types of reactions. The
halogen can be reduced off most effectively using lithium or zinc metal. This
procedure works best with bromides and iodides.
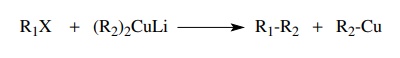
Alternatively
alkyl halides undergo coupling reactions with lithium organ-ocuprates (which
are prepared from alkyl halides) to give alkanes by carbon – carbon bond
formation. Other metals can be used to promote the same kind of coupling, but
the use of cuprates is the most efficient and general.
It
is clear that there are many different ways to carry out the installation of a
particular functional group in a molecule. The ones discussed here are often
the most general and practical, and they are often the first ones tried in the
laboratory. However, it is also common that a particular substrate will not
give good results with any of the common reagents. For this reason new methods
of functional group manipulation are constantly being sought that are even more
general, milder, more selective, cheaper, easier, and use more readily
available starting materials than other methods.
