Alcohols
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Functional Group Synthesis
The alcohol functional group is a very important functional group in organic chemistry. Not only do many important compounds and pharmaceuticals contain the alcohol group, but the alcohol group can be prepared from many other groups and converted to many functional groups.
ALCOHOLS
The
alcohol functional group is a very important functional group in organic
chemistry. Not only do many important compounds and pharmaceuticals contain
the alcohol group, but the alcohol group can be prepared from many other groups and converted to many functional groups. Thus alcohols occupy a central position
in functional group manipulations. In addition, most of the tradi-tional
methods for the preparation of alcohols are still among the most useful
methods.
Alcohols
are at a fairly low oxidation level compared to other oxygen-containing functional
groups and consequently are readily prepared by reduction. Large numbers of
reductive methods have been reported for the preparation of alcohols.
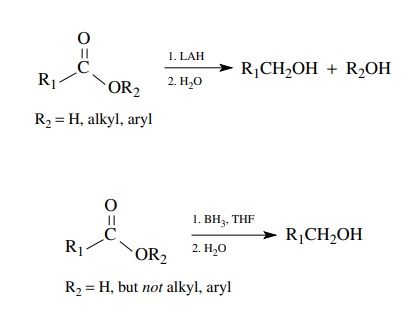
Carboxylic acids and esters react vigorously with lithium aluminum hydride
(LAH) to produce primary alcohols. Carboxylic acids, but not esters, are also
reduced easily by borane, which is the only reducing agent that reacts faster
with carboxylic acids than with esters or other acid derivatives.
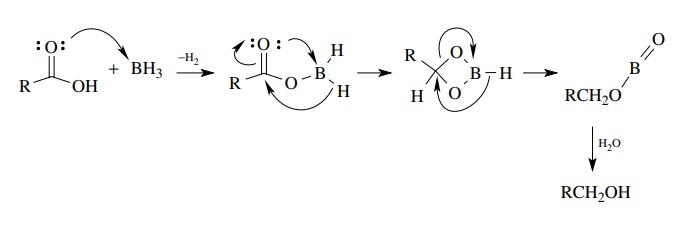
Its
unique reactivity comes from the fact that borane first forms a Lewis acid –
base complex with the acid and then a boron – carboxylate intermediate which
increases the reactivity of the boron hydride and delivers the hydride by an
intramolecular reaction. As such it provides a selective way to reduce acids
and produce alcohols in the presence of most other functional groups.
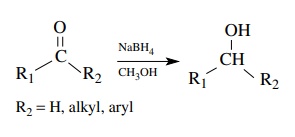
Aldehydes
and ketones are conveniently reduced by sodium borohydride, which is much
milder than LAH and does not require aprotic conditions (an alcohol is often
the preferred reaction solvent). Aldehydes give primary alcohols while ketones
give secondary alcohols.
Alcohols
and olefins are at the same oxidation level and are interconvertible without a
change in oxidation level. Addition of water across an olefinic dou-ble bond is
thus a common method for the preparation of alcohols. However, simple
acid-catalyzed addition of water is often the least desirable alternative.
Instead methods are used which permit much greater regiochemical control and
are milder. For example, the preferred way to produce the Markovnikov alcohol
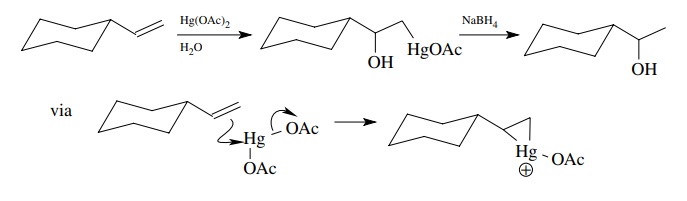
from an olefin is to use hydroxymercuration for the addition step followed by
reductive removal of mercury with NaBH4. In this process mercury
serves as a surrogate for the proton during the addition step and is replaced
by hydrogen in the reduction. The advantage is that the mercuric electrophile
forms a bridged or partially bridged intermediate that is stabilized and hence
gives higher yields and cleaner product mixtures because rearrangements are
suppressed. Other nucle-ophiles such as alcohols, acids, and hydrogen peroxide
can also be employed as the cation trap.
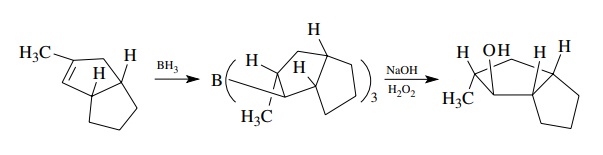
Hydroboration
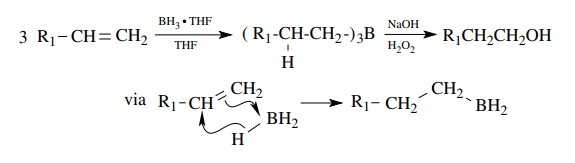
is widely employed to obtain an anti-Markovnikov alcohol from an olefin.
Addition of diborane to the double bond produces an organoborane intermediate.
Three equivalents of the olefin are needed to consume the BH3 and a
trialkylborane is produced. Reaction with basic H2O2
converts the carbon – boron bond to a carbon oxygen bond. This process is
effective and widely used.
The
initial addition step occurs in a concerted fashion so that the hydrogen and
boron are added to the same side of the planar double bond. Furthermore, the
cleavage of the carbon – boron bond occurs with retention of configuration.
These features can be used advantageously to prepare stereochemically pure
alcohols.