Acid-Base Equilibria
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Acidity and Basicity
Since pKa values always refer to the ionization of an acid in water under standard conditions (dilute aqueous solution at 25◦ C), they can be used to predict the positions of acid–base equilibria.
ACID–BASE EQUILIBRIA
Since
pKa values always refer to
the ionization of an acid in water under standard conditions (dilute aqueous
solution at 25◦
C), they can be used to predict the positions of acid–base equilibria. The
position of the following equilibrium (pKeq)
can be calculated by noting that in going from left to right isopropanol is
acting as an acid.
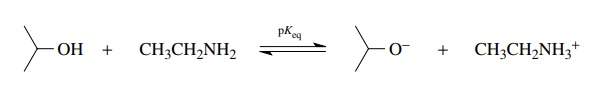
In
going in the reverse direction, from right to left, the ethyl ammonium ion is
acting as an acid. Having identified the compounds acting as acids on either
side of the equilibrium, the pKa
values for those acids are found and added to the equation.
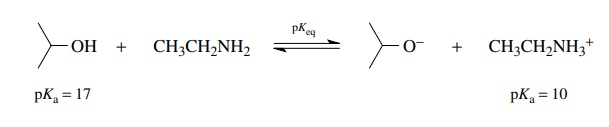
To
evaluate the position of the equilibrium, pKeq
is determined by the equation pKeq
= pKa (reactant acid) −pK
a (product acid). For the above example pKeq =
17 − 10 = 7, and based on the definition of pK , Keq
= 10−7 for this
equi-librium. Thus the equilibrium lies far to the reactant side; that is, very
little isopropoxide ion or ethyl ammonium ion is present at equilibrium. This
tech-nique is applicable for virtually any acid–base equilibrium. The three
required steps are to (a) write a balanced equation that describes the
equilibrium to be analyzed, (b) identify the species which is acting as an acid
on each side of the equilibrium and write down its pKa, and (c) subtract the pKa of the prod-uct acid from the pKa of the reactant acid to give the pK eq for the equilibrium in
question. It is a requirement that the pKa’s
of the acids on each side of the equilibrium are known or can be estimated
reasonably well. Furthermore, the pKeq
that is determined refers to the equilibrium in the direction it is writ-ten. It is therefore important to write
the chemical equilibrium as you wish to analyze
it.
The
reaction of methyl lithium with water is an acid–base reaction. Going from left
to right, water donates a proton to CH3Li so it functions as the
acid on the left. Going from right to left, methane donates a proton to LiOH so
it functions as the acid on the right. The pKa’s
of the acids on either side of the equilibrium reaction are subtracted and pKeq = −34.3. Thus the equilibrium constant is Keq = 1034.3, which shows that the equilibrium lies very far to the
right—so far to the right that for all practical purposes the conversion is
quantitative.
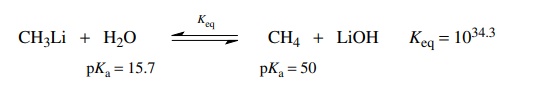
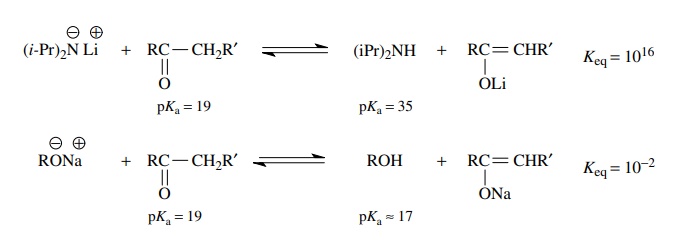
It
is now common experimental practice to react ketones with lithium diiso-propyl
amide (LDA) in order to generate the enolate of the ketone. This methodol-ogy
has largely replaced the older approach to enolates, which employed alkoxide
bases to remove a proton alpha to the carbonyl group. Comparison of the
equilib-rium constants for these two acid–base reactions reveals why the LDA
method is preferable. The use of the amide base leads to essentially complete
conversion of the ketone to its enolate (Keq
≈ 1016).
At equilibrium, there is virtually no unreacted ketone present in solution,
only diisopropyl amine and the enolate, which can subsequently react cleanly
and controllably with electrophiles which are added to the reaction mixture. In
contrast, the use of an alkoxide base results in only partial conversion of the
ketone to its enolate (Keq
≈ 10−2) so that at
equilibrium the enolate, even greater amounts of the unreacted ketone, and some
unreacted alkoxide are present in the reaction mixture. It is therefore
difficult to react the enolate with an added electrophile while avoiding
competing reac-tions of the enolate with the unreacted ketone and the alkoxide
with the added electrophile. Thus control of the reaction is difficult and
consequently yields are decreased and the product mixture is more complex.
The
ability to make good estimates of acid–base equilibrium constants is an
invaluable aid in thinking about organic reactions and processes. Moreover,
experimental workup procedures often require pH control that can be easily
understood on the basis of pKa
considerations. Thus the concept of acid strength is exceedingly important and
should be mastered.
A
similar development can be used for the quantitation and comparison of the base
strengths of organic bases (the ability to accept protons from acids). To do
this, Kb is defined as
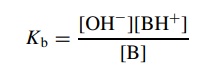
the
fraction of organic base which removes a proton from the standard acid—water.
A
scale of Kb’s was
developed with which to compare base strengths quantitatively.
The
situation can become quite confusing since two sets of constants are defined, Ka and Kb. It
turns out that acidities and basicities are inversely related by the ionization constant of water. That is,
the stronger an acid is in donating a proton to water, the weaker its conjugate
base is in removing a proton from water. This seems eminently reasonable since
if something gives up a proton easily, then it should not take protons back
easily. Put in more chemical terms, strong acids have weak conjugate bases, and
weak acids have strong conjugate bases. It has become the convention to list
only pKa’s as a measure of
both acidity and basicity. The only
thing to remember is to assign the appropriate Ka to the reaction in the “acidic” direction.
For
example, the base strengths of NH3 and CH3O− can be compared in
two different ways. First, the reactions of these two bases with water can be
written as follows, and by the preceding analysis the two equilibrium constants
can be estimated by identifying the acids on either sides of the two equilibria
and subtracting their pKa’s.

It
is seen that the equilibrium for the reaction of methoxide with water lies much
farther to the right (Keq = 10) than the reaction of ammonia
with water (Keq = 10−6). Clearly methoxide
is much better at removing a proton from water than is ammonia by about 10 .
Therefore methoxide is a stronger base by about 107 than ammonia.
Alternatively it is noted that the conjugate acids of ammonia and methoxide are the ammonium ion and methanol, respectively, and the equations for their ionization in water are
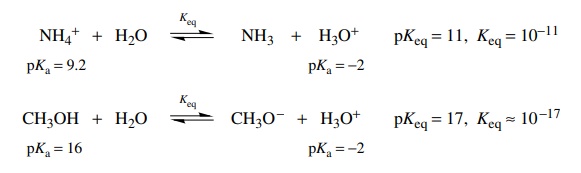
Comparing
these two equilibria, it is seen that methanol is a much weaker acid than
ammonium ion by about 107 (i.e., the pKa of methanol is larger by about seven logarithm units
than the pKa of ammonium);
thus its conjugate base methoxide should be a much stronger base than ammonia
by 107. Either method of analyzing the situation is acceptable, and
each gives the same relative basicities without the need for two sets of ionization
constants, Ka and Kb.
Related Topics
