Acetylation
| Home | | Biopharmaceutics and Pharmacokinetics |Chapter: Biopharmaceutics and Pharmacokinetics : Biotransformation of Drugs
This reaction is basically an acylation reaction and thus similar to conjugation with α-amino acids.
ACETYLATION
This reaction is basically an acylation reaction and thus similar to conjugation with α-amino acids. The analogy also lies in the fact that both reactions yield amide products. Acetylation however differs from α-amino acid conjugation in that the substrates are exogenous amines (and not carboxylic acids) and the acylating agent is endogenous acetyl CoA (CH3COSCoA).
The general sequence of reaction is similar to that for α-amino acid conjugation. The enzyme involved is the nonmicrosomal N-acetyl transferase.
Acetylation is an important metabolic pathway for
drugs containing primary amino groups. Alcohols (e.g. choline) and thiols (e.g.
CoASH) also undergo acetylation but only the endogenous ones.
Examples of drugs undergoing acetylation are –
Primary aliphatic amines e.g.
histamine, mescaline.
Primary aromatic amines e.g. procainamide, PAS,
PABA, dapsone.
Sulphonamides e.g. sulphanilamide, sulphapyridine.
Hydrazines/hydrazides e.g. hydralazine, isoniazid,
phenelzine.
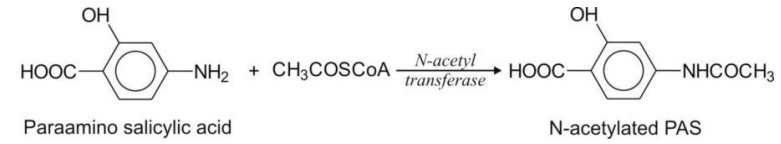
Acetylation may sometimes lead to toxic products,
e.g. acetyl derivatives of some sulphonamides (cause renal toxicity due to
decreased water solubility of the metabolites formed) and reactive
arylacetamides.
One of the interesting facts about acetylation is
pharmacogenetic difference in the rate at which it proceeds in man, (called as acetylation polymorphism). The
distribution of population in acetylating certain substrates is bimodal viz. slow acetylator and rapid acetylator phenotypes. As a result, large inter-ethnic group
variations in the therapeutic and toxic
levels of drugs that undergo acetylation have been observed, e.g. isoniazid.
Related Topics
