β-Lactamase inhibitors
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : Antibiotics And Synthetic Antimicrobial Agents: Their Properties And Uses
The strategy of protecting vulnerable penicillins against enzymemediated hydrolysis by combining them with a β-lactamase inhibitor resulted in the first such combination product, coamoxiclav, in 1981.
β-LACTAMASE INHIBITORS
The strategy of protecting vulnerable
penicillins against enzymemediated hydrolysis by combining them with a
β-lactamase inhibitor resulted in the first such combination product,
coamoxiclav, in 1981. Table 11.1 shows
that penicillins with β-lactamase inhibitors represent approximately 6.5% of
the antibiotics prescribed to UK outpatients in the European hospital survey,
and coamoxiclav, which consists of amoxicillin plus clavulanic acid, is by far
the most important combination available. Clavulanic acid has also been used to
protect ticarcillin from β-lactamase attack, and two penicillanic acid sulphones,
sulbactam and tazobactam, have been used to protect ampicillin and piperacillin
respectively. In each case the protecting molecule is itself a β-lactam
antibiotic, but one possessing little antimicrobial activity in its own right.
The benefit afforded is an extension of the antimicrobial spectrum of the
antibiotic receiving protection; this is achieved by negating the effects of
β-lactamases produced by staphylococci and some Gram-negative species, which
would otherwise be resistant. In the case of coamoxiclav for example, the
combination exhibits activity not only against many strains of Staph. aureus, but also against strains of E. coli, H. influenzae and Klebsiella species, against which amoxicillin
alone would be ineffective. This means that coamoxiclav should, in theory, be
reserved for infections known, or likely, to be due to amoxicillin-resistant
β-lactamase-producing strains, but unfortunately it is not always used in this
prudent manner.
Clavulanic acid was isolated from Streptomyces clavuligerus and belongs to a class
of β-lactams termed clavems, which differ from penicillins in two respects:
namely the replacement of sulphur in the penicillin thiazolidine ring (Figure 11.1)
with oxygen in the clavam oxazolidine ring (Figure 11.5A),
and the absence of the side chain at position 6. Clavulanic acid also affords
some protection against enzymes that are primarily active against
cephalosporins rather than penicillins, but this protection is quite modest
compared with its activity against ‘penicillinases’.
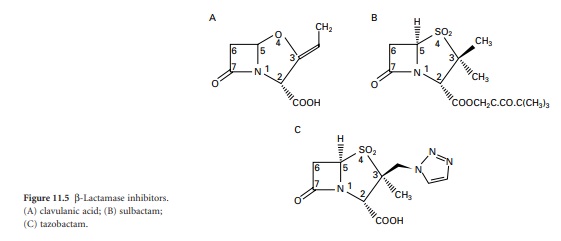
Sulbactam and tazobactam (Figure 11.5B and C) can be
regarded as β-lactam molecules that resemble penicillins except that the
sulphur atom of the thiazolidine ring is converted to a sulphone, and again,
there is no side chain at position 6. Sulbactam is effective against a similar
range of β-lactamases to clavulanic acid, although it is not quite as potent.
In both cases, the range of enzymes does not normally include those
manufactured by Ps. aeruginosa and other
problem Gramnegative organisms. Sulbactam has been combined with both
ampicillin and cefoperazone, but in both products the two ingredients were
separate entities; this poses the potential problem that their pharmacokinetics
might not perfectly match, so that the two agents might not appear at the
infection site in the optimal concentration ratio at the same time. In the case
of sulbactam and ampicillin this problem was partly overcome by covalently
linking the two molecules to create sultamicillin which is well absorbed
following oral administration and then hydrolysed to liberate equimolar proportions
of the individual components.
